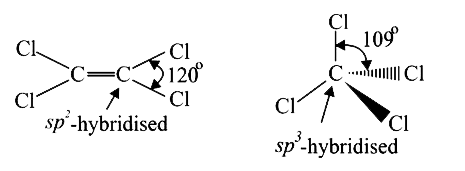A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Advanced (Archive)
- sp^(3) hybrid orbitals have equal s and p characters .
Text Solution
|
- In benzene, carbon uses all the three p-orbitals for hybridisation .
Text Solution
|
- The Cl-C-Cl angle is 1,1,2,2-tetrachloroethene and tetrachloromethane ...
Text Solution
|
- The molecule that has linear structure is
Text Solution
|
- The species in which the central atom uses sp^(2) hybrid orbitals in i...
Text Solution
|
- The molecule which has pyramidal shape is
Text Solution
|
- Which of the following is paramagnetic ?
Text Solution
|
- The molecule which has zero dipole momnet is .
Text Solution
|
- The valence atomic orbital on C in silver acetylide is ……………hybridised...
Text Solution
|
- Between Na^(+) and Ag^(+) , which is stronger Lewis acid and why?
Text Solution
|
- The shape of [CH(3)]^(o+) is .
Text Solution
|
- The presnce of polar bonds in a polyatomic molecule suggests that the ...
Text Solution
|
- What effect should the following resonance of vinyl chloride have on i...
Text Solution
|
- Arrange the following as stated Increasing strength of hydrogen bond...
Text Solution
|
- In the reaction I^(-) +I(2) rarr I(3)^(-), the Lewis acid is ……….. .
Text Solution
|
- The linear structure is assumed by .
Text Solution
|
- Arrange the following ions in order of their increasing size: Li^(+), ...
Text Solution
|
- The maximum number of H-bonds a water molecule can form are
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(2)^(-) is
Text Solution
|
- Which of the folowing have identical bond orders ? .
Text Solution
|