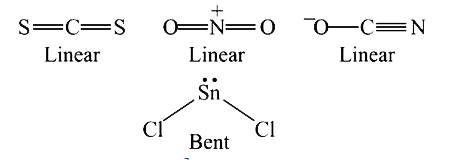
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Advanced (Archive)
- Arrange the following as stated Increasing strength of hydrogen bond...
Text Solution
|
- In the reaction I^(-) +I(2) rarr I(3)^(-), the Lewis acid is ……….. .
Text Solution
|
- The linear structure is assumed by .
Text Solution
|
- Arrange the following ions in order of their increasing size: Li^(+), ...
Text Solution
|
- The maximum number of H-bonds a water molecule can form are
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(2)^(-) is
Text Solution
|
- Which of the folowing have identical bond orders ? .
Text Solution
|
- The dipole moment of CH3F is greater than that of CH3Cl.
Text Solution
|
- H(2)O moleule is linear
Text Solution
|
- The dipole moment of KCI is 3.36 xx 10^(-29)Cm The interatomic distanc...
Text Solution
|
- The two types of bonds pressent in B(2)H(6) are covalent and .
Text Solution
|
- All molecules with polar bonds have dipole moment.
Text Solution
|
- Explain the difference in the nature of bonding in LiF and LiI .
Text Solution
|
- Among the following species identify the isostructural pairs NH(3), NO...
Text Solution
|
- Which of the following molecules is planar ? .
Text Solution
|
- The number and type of bonds between two carbon atoms in C(2) are:
Text Solution
|
- Fill in the blanks by chossing the appropriate word/words from those g...
Text Solution
|
- Among N(2)O,SO(2),I(3)^(o+) and I(3)^(Theta) the linear species are an...
Text Solution
|
- Which one of the following compounds has sp^(2) hybridisation ? .
Text Solution
|
- Among KO(2) , AlO(2)^(+) , BaO(2) and NO(2)^(+) unpaired electron is p...
Text Solution
|