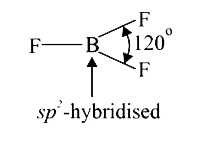
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Advanced (Archive)
- Explain the difference in the nature of bonding in LiF and LiI .
Text Solution
|
- Among the following species identify the isostructural pairs NH(3), NO...
Text Solution
|
- Which of the following molecules is planar ? .
Text Solution
|
- The number and type of bonds between two carbon atoms in C(2) are:
Text Solution
|
- Fill in the blanks by chossing the appropriate word/words from those g...
Text Solution
|
- Among N(2)O,SO(2),I(3)^(o+) and I(3)^(Theta) the linear species are an...
Text Solution
|
- Which one of the following compounds has sp^(2) hybridisation ? .
Text Solution
|
- Among KO(2) , AlO(2)^(+) , BaO(2) and NO(2)^(+) unpaired electron is p...
Text Solution
|
- CN^(Theta) and N(2) are isoelectronic But in contrast to CN^(Theta),N(...
Text Solution
|
- Which contains both polar and non-polar bonds ? .
Text Solution
|
- Assertion (A) : LiCl is predominantly a covalent compound. Reason (R...
Text Solution
|
- Statement I: The electronic structure of O(3) is : Statement II...
Text Solution
|
- Explain the non-linear shape of H(2)S and non-planar shape of PCl(3) u...
Text Solution
|
- Using the VSEPR theory, identify the type of hybridization and draw th...
Text Solution
|
- The geometry and the type of hybrid orbital present about the central ...
Text Solution
|
- In compounds of type ECl(3), where E=B,P. As or Bi, the angles Cl-E-Cl...
Text Solution
|
- The corrrect order of C-O bond length among CO, CO3^(2-), CO2 is
Text Solution
|
- In overset(1)CH(2)=overset(2)CH-overset(3)CH(2)-overset(4)C-=overset(5...
Text Solution
|
- The geometry of H2S and its dipole moment are
Text Solution
|
- Write the molecular orbital electron distribution of oxygen (O(2)) Spe...
Text Solution
|