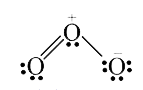
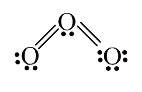
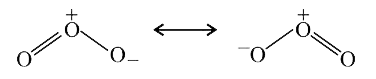
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Advanced (Archive)
- Which contains both polar and non-polar bonds ? .
Text Solution
|
- Assertion (A) : LiCl is predominantly a covalent compound. Reason (R...
Text Solution
|
- Statement I: The electronic structure of O(3) is : Statement II...
Text Solution
|
- Explain the non-linear shape of H(2)S and non-planar shape of PCl(3) u...
Text Solution
|
- Using the VSEPR theory, identify the type of hybridization and draw th...
Text Solution
|
- The geometry and the type of hybrid orbital present about the central ...
Text Solution
|
- In compounds of type ECl(3), where E=B,P. As or Bi, the angles Cl-E-Cl...
Text Solution
|
- The corrrect order of C-O bond length among CO, CO3^(2-), CO2 is
Text Solution
|
- In overset(1)CH(2)=overset(2)CH-overset(3)CH(2)-overset(4)C-=overset(5...
Text Solution
|
- The geometry of H2S and its dipole moment are
Text Solution
|
- Write the molecular orbital electron distribution of oxygen (O(2)) Spe...
Text Solution
|
- Draw the molecular structures of XeF(2), XeF(4) and XeO(2)F(2), indica...
Text Solution
|
- Which of the following statement is correct among the species CN^(Θ),C...
Text Solution
|
- Molecular shape of XeF(2), BeF(2) and CF(2) are :
Text Solution
|
- Nodal planes of pi-bonds in CH(2)=C=C=CH(2) are located in,
Text Solution
|
- The hybridization of atomic orbitals of nitrogen in N(3)^(-),(H(3)Si)(...
Text Solution
|
- Specify the coordination geometry around and hybridisation of N and B ...
Text Solution
|
- Identify the least stable ion amongst the following:
Text Solution
|
- Which of the following molecular species has unpaired electrons(s) ? .
Text Solution
|
- Which of the following molecules has highest dipole moment?
Text Solution
|