Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Advanced (Archive)
- The number of lone piar(s) in XeOF(4) is .
Text Solution
|
- One the basic of ground electronic configuration, arrange the followin...
Text Solution
|
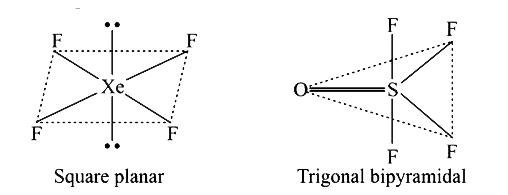
- Draw the shape of XeF(4) and OSF(4) according to VSEPR theory Show the...
Text Solution
|
- According to MO theory .
Text Solution
|
- Predict whether the following molecules are isostructural or not Justi...
Text Solution
|
- Which species has the maximum number of lone pair of electrons on th...
Text Solution
|
- Among the following the paramagnetic compound is
Text Solution
|
- The species having bond order differnet from that in CO is .
Text Solution
|
- Match each of the diatomic molecules in Column I with its property/pro...
Text Solution
|
- Assuming that Hund’s rule is violated, the bond order and magnetic nat...
Text Solution
|
- Based on VSEPR theory the number of 90^(@) F -Br -F angles in a molecu...
Text Solution
|
- The species having pyramidal shape is
Text Solution
|
- Which one of the following molecules is expected to exhibit diamagneti...
Text Solution
|
- Assuming 2s-2p mixing is NOT operative, the paramagnetic species among...
Text Solution
|
- Hydrogen bonding plays a central role in the following phenomena .
Text Solution
|
- Match the orbital overlap figure in List I with the description given ...
Text Solution
|
- Among the triatomic molecules/ions, BeCI(2), N(3)^(Theta) ,N(2)O , ...
Text Solution
|
- The compound(s) with two lone pairs of electron on the central atom is...
Text Solution
|
- According to Molecular Orbital Theory,
Text Solution
|
- The sum of the number of lone pair of electrons on each central atom i...
Text Solution
|