Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
VMC MODULES ENGLISH|Exercise LEVEL -0 (Short Answer Type-II)|27 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise Level-1|75 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-I|10 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-LEVEL -0 ( Short Answer Type)
- An iron cylinder contains helium at a pressure of 250 k Pa at 300 K. T...
Text Solution
|
- A large flask fitted with a stop-cock is evacuated and weighted, its m...
Text Solution
|
- Calculate the pressure exerted by 110 g of carbon dioxide in a vessel ...
Text Solution
|
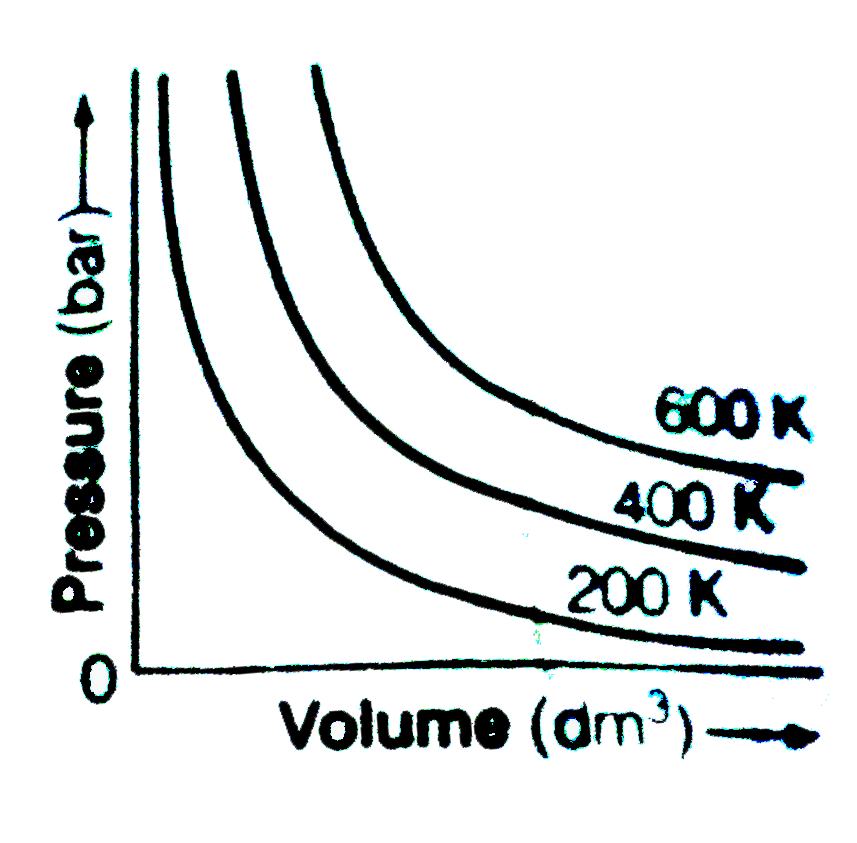
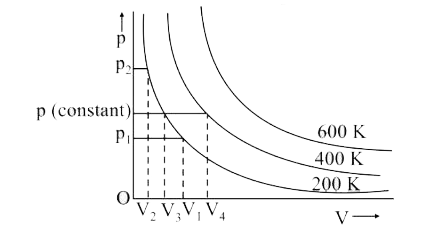
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- The variation of pressure with volume of the gas at different temperat...
Text Solution
|
- Why does the boundary between liquid phase and gaseous phase disappear...
Text Solution
|
- Explain the term 'laminar flow'. Is the velocity of molecules same in ...
Text Solution
|
- Assuming the same pressure in each case calculate the mass of hydrogen...
Text Solution
|