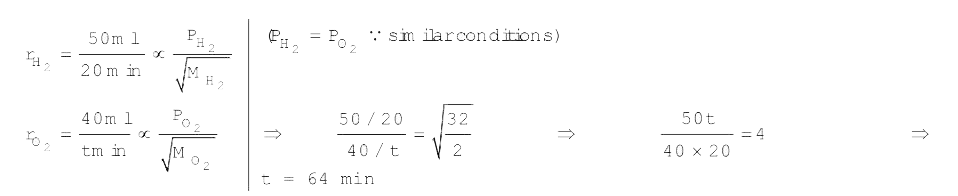A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
VMC MODULES ENGLISH|Exercise Level-2|50 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise Level-2 (NUMBERICAL VALUE TYPE FOR JEE MAIN|15 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise LEVEL -0 (Short Answer Type-II)|27 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-Level-1
- At what temperature will the molar kinetic energy of 0.3 mol of He be ...
Text Solution
|
- The molecular velocities of two gases at same temperature are u(1) and...
Text Solution
|
- 50 mL of hydrogen diffuses out through a small hole from a vessel in 2...
Text Solution
|
- The average kinetic energy of an ideal gas per molecule in SI units at...
Text Solution
|
- KE per unit volume is:
Text Solution
|
- Select correct statement(s):
Text Solution
|
- Select correct statement(s)
Text Solution
|
- The expression of average speed of molecules of a gas is given as :
Text Solution
|
- For a given gas, which of the following relationships is correct at a ...
Text Solution
|
- Which of the following is expected to possess the largest root mean sq...
Text Solution
|
- The RMS velocity of hydrogen is sqrt7 times the RMS velocity of nitrog...
Text Solution
|
- The density of a gas at 27^(@)C and 1 atm is d. At what temperature w...
Text Solution
|
- A certain gas effuses through a small opening of a vessel at a rate wh...
Text Solution
|
- The weight of CH(4) in a 9L cylinder at 27^(@)C temperature and 16 at...
Text Solution
|
- The ratio of most probable speed, average speed and rms speed of gas m...
Text Solution
|
- Equal weights of methane and hydrogen are mixed in an empty container ...
Text Solution
|
- A gas cylinder containing cooking gas can withstand a pressure of 14....
Text Solution
|
- At what temperature do the average speed of CH(4)(g) molecules equal t...
Text Solution
|
- Which of the following expressions is correct ?
Text Solution
|
- Consider the reaction 2Al (g) + 3Cl(2) (g) rArr 2Al Cl(3) (g). The app...
Text Solution
|