A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
VMC MODULES ENGLISH|Exercise Level-2|50 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise Level-2 (NUMBERICAL VALUE TYPE FOR JEE MAIN|15 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise LEVEL -0 (Short Answer Type-II)|27 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-Level-1
- At a high pressure, the compressibility factor (Z) of a real gas is us...
Text Solution
|
- At a constant pressure, what should be the percentage increase in the ...
Text Solution
|
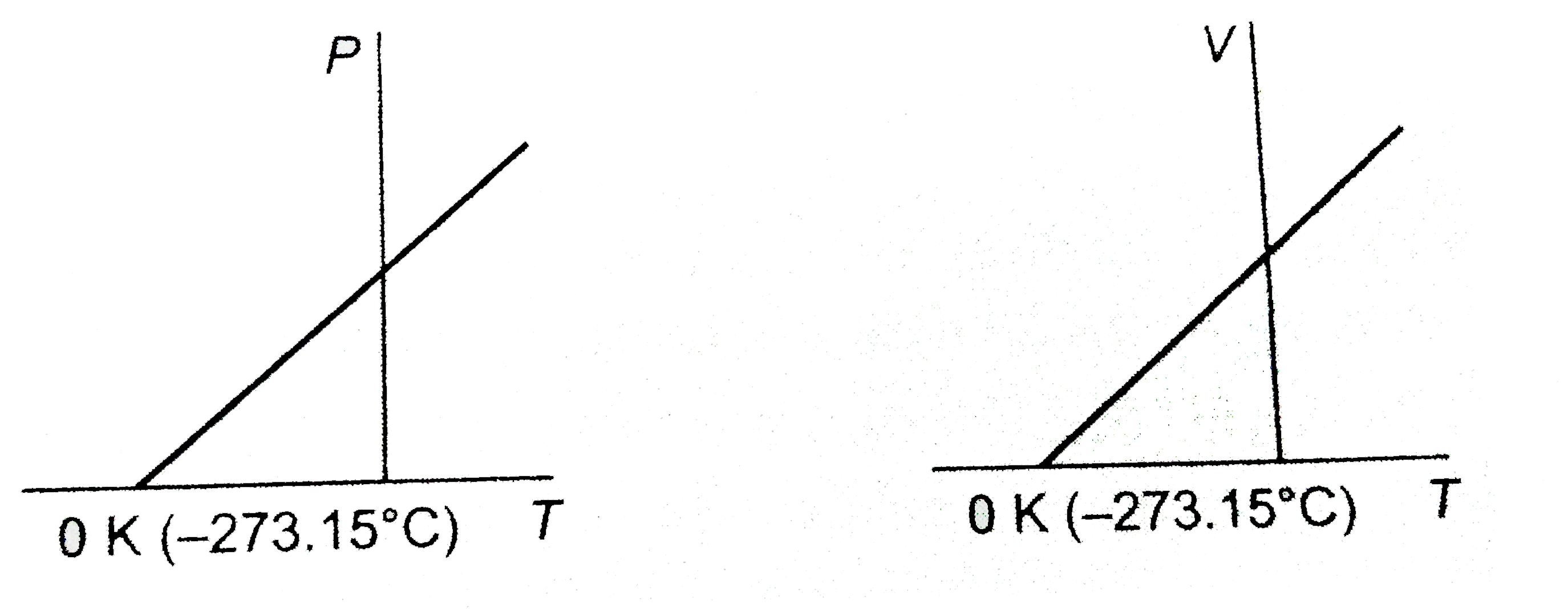
- What conclusion would you draw from the following graphs for an ideal ...
Text Solution
|
- Which of the following represents the van der Walls equation for n mol...
Text Solution
|
- Which of the following equations represents the compressibility factor...
Text Solution
|
- At high pressure , the van der Waals equation is reduced to
Text Solution
|
- The Boltzmann constant k is given by k = ………..
Text Solution
|
- Units of van der Waal's constants 'a' and 'b' are respectively
Text Solution
|
- Which of the following gas will have highest value of van der Waal's c...
Text Solution
|
- The Boyle temperature for real gases is given by :
Text Solution
|
- A 4.40 g piece of solid CO(2) (dry ice) is allowed to sublime in a bal...
Text Solution
|
- A He atom at 300 K is released from the surface of the earth to travel...
Text Solution
|
- An ideal gas obeying the kinetic theory of gases can be liquefied if
Text Solution
|
- The pressure of a real gas is less than the pressure of an ideal gas b...
Text Solution
|
- Distribution of molecules with velocity is represented by the curve as...
Text Solution
|
- A ballon filled with ethyne is pricked with a sharp point and quickly ...
Text Solution
|
- If X(M), X(P), and X(V) are mole fraction, pressure fraction and volum...
Text Solution
|
- A 100 mL flask contained H(2) at 200 Torr, and a 200 mL flask containe...
Text Solution
|
- Ratio of the rate of diffusion of He to H(2) at 0^(@)C is same in th...
Text Solution
|
- Which one of the following statement is not true about the effect of a...
Text Solution
|