A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
VMC MODULES ENGLISH|Exercise JEE-Advanced|78 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise ILLUSTRATION|30 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise Level-2 (NUMBERICAL VALUE TYPE FOR JEE MAIN|15 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-JEE-Main ( ARCHIVE )
- Equal mass of methane and oxygen are mixed in an empty container at 2...
Text Solution
|
- The rate of diffusion of methane at a given temperature is twice that ...
Text Solution
|
- Equal weights of ethane and hydrogen are mixed in an empty container a...
Text Solution
|
- One mole of nitrogen gas at 0.8 atm takes 38 s to diffuse through a pi...
Text Solution
|
- The pressure exerted by 12 g of an ideal gas at temperature t^(@)C in ...
Text Solution
|
- For gaseous state, if most probable speed is denoated by C^(**), avera...
Text Solution
|
- If Z is a compressibility factor, van der Waals equation at low pressu...
Text Solution
|
- Which one of the following is not an assumption in the kinetic theory ...
Text Solution
|
- Under which of the following conditions applied together, a gas deviat...
Text Solution
|
- Two closed bulbs of equal volume (V) containing an ideal gas initially...
Text Solution
|
- Among the following, the incorrect statement is :
Text Solution
|
- At 300 K, the density of a certain gaseous molecule at 2 bar is double...
Text Solution
|
- Assuming ideal gas behaviour, the ratio of density of ammonia to that ...
Text Solution
|
- An open vessel at 27^@C is heated untill two fifth of the air (assumed...
Text Solution
|
- Consider the van der Waals' constants, a and b, for the following gase...
Text Solution
|
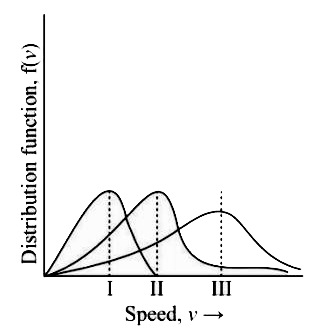
- Points I, II and III in the following plot respectively correspond to ...
Text Solution
|
- At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate...
Text Solution
|