Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
VMC MODULES ENGLISH|Exercise ILLUSTRATION|30 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise SOLVED EXAMPLES|20 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise JEE-Main ( ARCHIVE )|17 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-JEE-Advanced
- 4.215 g a metallic carbonate was heated in a hard glass tube and CO2 e...
Text Solution
|
- 3.7 g of a gas at 25°C occupies the same volume as 0.184 g of hydrogen...
Text Solution
|
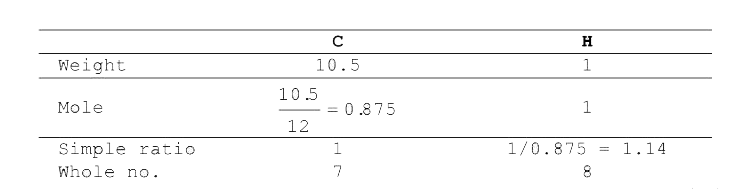
- A hydrocarbon contains 10.5 g of carbon per gram of hydrogen. 1 L of v...
Text Solution
|
- The pressure in a bulb dropped from 2000 to 1500 mm Hg in 47 min when ...
Text Solution
|
- The ratio of root mean square velocity to average velocity of a gas mo...
Text Solution
|
- Temperature at which gas behave ideally over a wide range of pressure ...
Text Solution
|
- Calculate the average kinetic energy in joules of the molecules in 8.0...
Text Solution
|
- Helium atom is two times heavier than a hydrogen molecule. At 289 K, t...
Text Solution
|
- A gas in a closed container will exert much higher pressure due to gra...
Text Solution
|
- Give reasons for the following in one or two sentences. (i) Equal vo...
Text Solution
|
- When 2g of a gas A is introduced into an evacuated flask kept at 25^@C...
Text Solution
|
- Oxygen is present in a 1 litre flask at a pressure of 7.6 xx 10^(-10) ...
Text Solution
|
- When an ideal gas undergoes unrestrained expansion, no cooling occurs ...
Text Solution
|
- Calculate the root mean square velocity of ozone kept in a closed vess...
Text Solution
|
- The rate of diffusion of a gas is
Text Solution
|
- Kinetic energy of a molecule is zero at 0^(@)C.T//F
Text Solution
|
- The rate of diffusion of a gas is………….proportional to both ……….. And s...
Text Solution
|
- The average speed of ideal gas molecule at 27^@C is 0.3 ms^-1 . Calcul...
Text Solution
|
- A spherical balloon of 21 cm diameter is to the filled up with H(2) at...
Text Solution
|
- The value of PV for 5.6 L of an ideal gas is ……… RT at NTP.
Text Solution
|