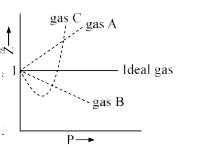
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
VMC MODULES ENGLISH|Exercise ILLUSTRATION|30 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise SOLVED EXAMPLES|20 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise JEE-Main ( ARCHIVE )|17 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-JEE-Advanced
- The average velocity of gas molecules is 400 m s^(-1). Calculate their...
Text Solution
|
- Positive deviation from ideal behaviour takes place because of
Text Solution
|
- The root mean square speed of one mole of a monoatomic gas having mole...
Text Solution
|
- The ratio of the rate of diffusion of helium and methane under indenti...
Text Solution
|
- The given graph represents the variations of compressibility factor Z...
Text Solution
|
- Match gases under specified condition listed in Column I with their pr...
Text Solution
|
- A gas described by van der Waals equation
Text Solution
|
- If a gas expands at constant temperature:
Text Solution
|
- At 400K,the root mean square (rms) speed of a gas X (molecular weight=...
Text Solution
|
- The term that corrects for the attractive forces present in a real gas...
Text Solution
|
- To an evacuated vessel with movable piston under external pressure of ...
Text Solution
|
- According to kinetic theory of gases:
Text Solution
|
- For one mole of a van der Waals gas when b = 0 and T = 300 K , the PV ...
Text Solution
|
- X and Y are two volatile liquids with molar weights of 10gmol^(-1) and...
Text Solution
|
- X and Y are two volatile liquids with molar weights of 10g mol^(-1) an...
Text Solution
|
- If the value of Avogadro numberis 6.023xx10^(23)mol^(-1) and the vaueo...
Text Solution
|
- One mole of a monoatomic real gas satisfies the equation p(V-b)=RT wh...
Text Solution
|
- The diffusion coefficient of an ideal gas is proportional to its mean ...
Text Solution
|
- A closed tank has two compartments A and B, both filled with oxygen (a...
Text Solution
|
- Which of the following is/are correct regarding root mean square speed...
Text Solution
|