Text Solution
Verified by Experts
Topper's Solved these Questions
THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise LEVEL - 0 Long Answer Type|13 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise LEVEL-1|75 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise LEVEL - 0 Short Answer Type - I|8 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|44 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMOCHEMISTRY-LEVEL - 0 Short Answer Type - II
- Calculate the bond energy of C - H bond, given that the heat of forma...
Text Solution
|
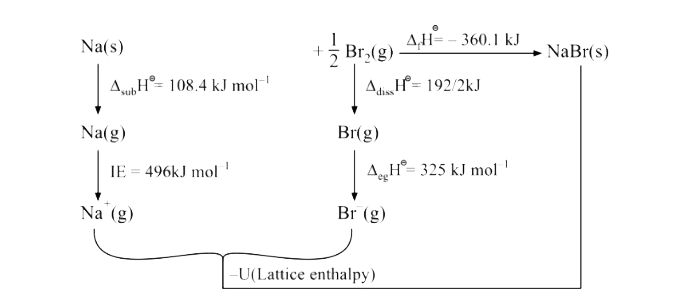
- Use the following data to calculate Delta("lattice") H^(Θ) " for " NaB...
Text Solution
|
- Show that the reaction CO(g) +(1//2)O(2)(g) rarr CO(2)(g) at 300K ...
Text Solution
|
- The heat of neutralization of (i) CHCl2 – COOH by NaOH is 12830 cal, (...
Text Solution
|
- A natural gas may be assumed to be a mixture of CH4 and C2H6 only. On ...
Text Solution
|
- The enthalpy of evaporation of water at 373 K is 40.67 kJ mol^(-1). Wh...
Text Solution
|