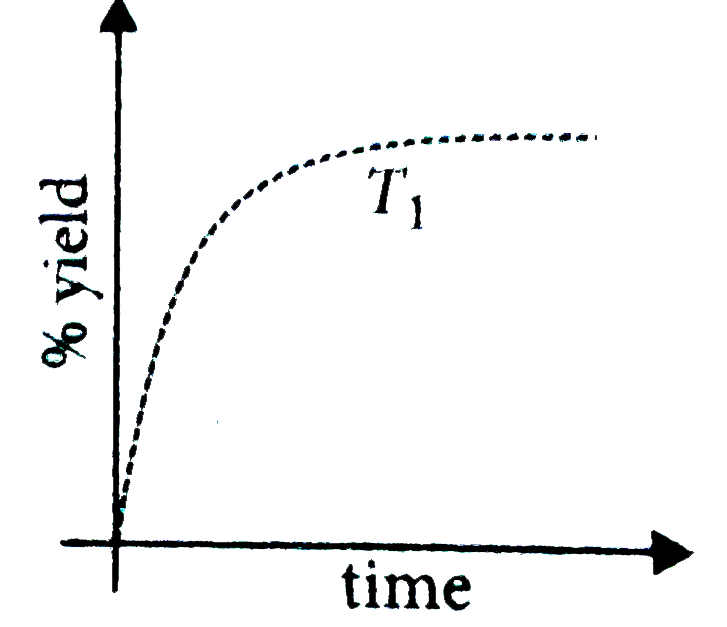
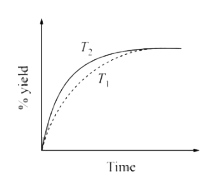
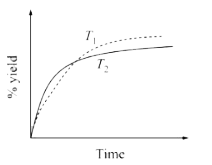
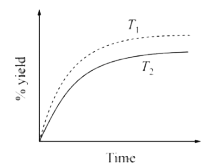
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise FUNDAMENTAL|49 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise ENABLE|49 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|24 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-JEE Advanced (Archive)
- N(2)O(4) is 25% dissociated at 37^(@)C and one atmosphere pressure. Ca...
Text Solution
|
- The equilibrium constant K(p) of the reaction: 2SO(2)+O(2) hArr 2SO(3)...
Text Solution
|
- For the reaction CO(g)+2H(2)(g)hArrCH(3)OH(g) Hydrogen gas is intr...
Text Solution
|
- For the reaction PCl(5)(g)hArrPCl(3)(g)+Cl(2)(g), the forward reaction...
Text Solution
|
- The rate of an exothermic reactions increases with increase in tempera...
Text Solution
|
- 0.15 mol of CO taken in a 2.5 L flask is maintained at 750 K alongwith...
Text Solution
|
- A 10-fold increase in pressure on the reaction N(2)(g)+3H(2)(g) hArr...
Text Solution
|
- For a gaseous reaction 2B rarr A, the equilibrium constant K(p) is ………...
Text Solution
|
- The degree of dissociation is 0.4 at 400 K and 1.0 atm for the gaseous...
Text Solution
|
- For the reversible reaction N(2)(g)+3H(2)(g) hArr 2NH(3)(g) at 500...
Text Solution
|
- At constant temperature , the equilibrium constant (KP) for the deco...
Text Solution
|
- Consider the following equilibrium in a closed container, N(2)O(4(g)...
Text Solution
|
- In the reaction equilibrium N(2)O(4) hArr 2NO(2)(g) When 5 mol of ...
Text Solution
|
- Ag^+ +NH3 hArr[Ag(NH3)]^+ K1=3.5xx10^-3 [Ag(NH3)]^+ +NH3 hArr[A...
Text Solution
|
- The thermal dissociation of equilibrium of CaCo(3)(s) is studied under...
Text Solution
|
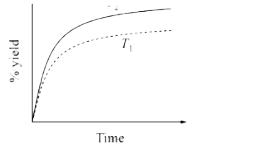
- The % yield of ammonia as a function of time in the reaction N(2(g))+3...
Text Solution
|
- The gas phase reaction 2NO(2)(g) rarr N(2)O(4)(g) is an exothermic rea...
Text Solution
|
- Delta G^(@) at 500 K for substance 'S' in liquid state and gaseous st...
Text Solution
|
- At a certain temperture in a 5L vessel 2 moles of carbon monoxide and ...
Text Solution
|
- For the following reaction, equilibrium constant K(c) at 298 K is 1.6x...
Text Solution
|