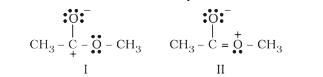A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
INTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise LEVEL 2 (Numerical value type )|15 VideosINTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE MAIN ARCHIVE|72 VideosINTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise level 1|73 VideosICONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosIONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - K|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-INTRODUCTION TO ORGANIC CHEMISTRY -LEVEL 2
- Which of the following is used for detection of amino acids during thi...
Text Solution
|
- Which of the following statements regarding the resonance energy of be...
Text Solution
|
- Which of the following two structures, I and II can be the major contr...
Text Solution
|
- In which compound delocalization is not possible-
Text Solution
|
- Which of the following species have a trigonal planar shape ?
Text Solution
|
- The IUPAC name of succinic acid is…………………
Text Solution
|
- The IUPAC name of CH(2)=CH-CH(CN)-CHO is :
Text Solution
|
- how many pi-electrons are there in CH(3)CN ?
Text Solution
|
- The tetracyanoethene contains :
Text Solution
|
- Tautomerism is not exhibited by :
Text Solution
|
- CH(2)=CHOH and CH(3)CHO are :
Text Solution
|
- The total number of possible isomeric trimethylbenzenes is
Text Solution
|
- The IUPAC name of BrCH(2)-CH(CONH(2))-CO-CH(2)-CH(2)-CH(3) is
Text Solution
|
- Which of the following is correct for
Text Solution
|
- What of the following compound is ( are ) named correctly ?
Text Solution
|
- The compound C(4)H(10)O shows "…................." Isomerism :
Text Solution
|
- C(8)H(10) has total number of isomers containing benzene ring equal to...
Text Solution
|
- Ethyl formate exhibits which of the following isomerism with Propanoic...
Text Solution
|
- CH(3)CH(2)NO(2) will show "" isomerism :
Text Solution
|
- Which of the following are isomers ?
Text Solution
|