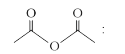
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
INTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise LEVEL 2 (Numerical value type )|15 VideosINTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE MAIN ARCHIVE|72 VideosINTRODUCTION TO ORGANIC CHEMISTRY
VMC MODULES ENGLISH|Exercise level 1|73 VideosICONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosIONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - K|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-INTRODUCTION TO ORGANIC CHEMISTRY -LEVEL 2
- The total number of possible isomeric trimethylbenzenes is
Text Solution
|
- The IUPAC name of BrCH(2)-CH(CONH(2))-CO-CH(2)-CH(2)-CH(3) is
Text Solution
|
- Which of the following is correct for
Text Solution
|
- What of the following compound is ( are ) named correctly ?
Text Solution
|
- The compound C(4)H(10)O shows "…................." Isomerism :
Text Solution
|
- C(8)H(10) has total number of isomers containing benzene ring equal to...
Text Solution
|
- Ethyl formate exhibits which of the following isomerism with Propanoic...
Text Solution
|
- CH(3)CH(2)NO(2) will show "" isomerism :
Text Solution
|
- Which of the following are isomers ?
Text Solution
|
- Write the structures of isomeric amines with molecular formula C(7)H(9...
Text Solution
|
- Which of the following compounds has chiral centre ?
Text Solution
|
- A nucleophile must necessarily have
Text Solution
|
- Arrange the following groups in their decreasing order of -I effect. ...
Text Solution
|
- Which one of the following carbocation is most stable ?
Text Solution
|
- Arraange the follwooing carbanions in decreasing order of their stabil...
Text Solution
|
- Which one of the following carbocations are most stable?
Text Solution
|
- Arrange the given carbocation in decreasing order of stability : ...
Text Solution
|
- Which one of the following is most stable carbocation ?
Text Solution
|
- Which one of the following carbanions is most stable ?
Text Solution
|
- Which one of the following statements is not correct ?
Text Solution
|