A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-INTRODUCTION TO ORGANIC CHEMISTRY -JEE ADVANCED ARCHIVE
- Draw the resonating structures of
Text Solution
|
- Glycerine contains one ......... Hydroxyl group.
Text Solution
|
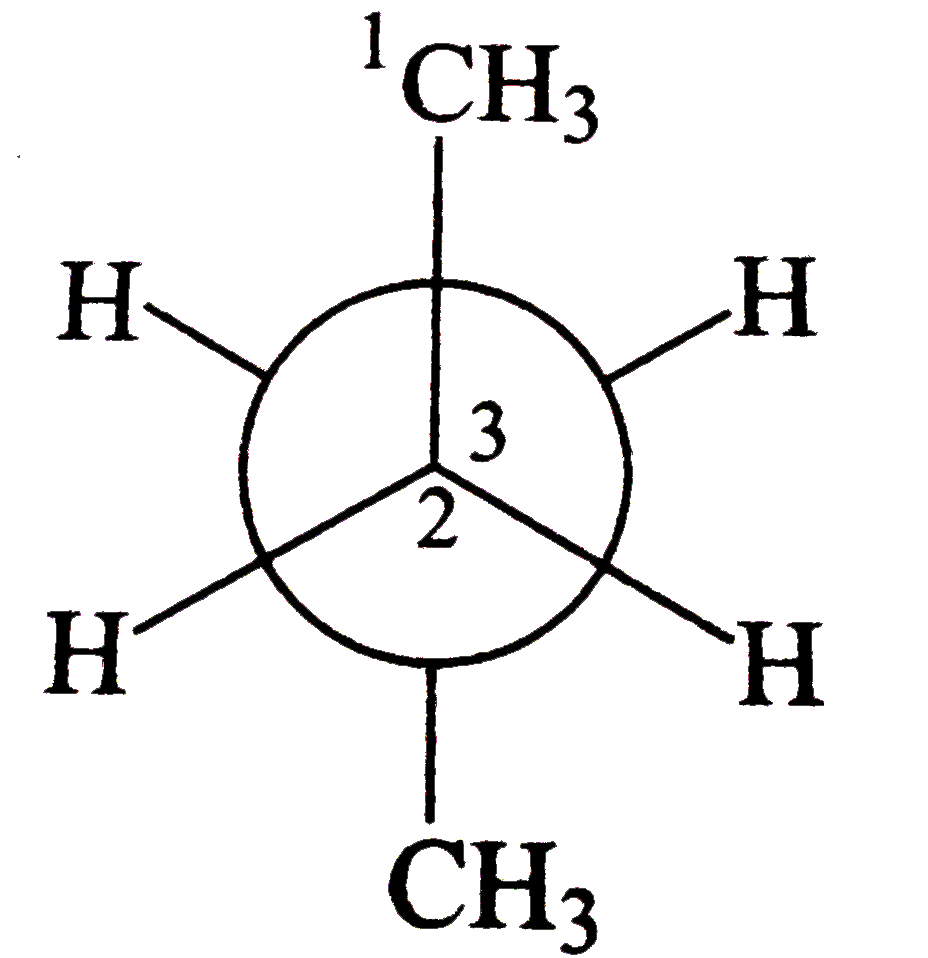
- C(2) is rotated anti-clockwise 120^(@) about C(2)-C(3) bond. The resul...
Text Solution
|
- Draw Newman projection of the less stable staggered from of butane. ...
Text Solution
|
- p-Hydroxybenzoic acid has a lower boiling point than o-hydroxybenzoic ...
Text Solution
|
- mu observed = Sigma mu(i)X(i) where mu(i) is the dipole moment of th...
Text Solution
|
- mu observed = Sigma mu(i)X(i) where mu(i) is the dipole moment of th...
Text Solution
|
- Which of the following resonating structures of 1-methoxy-1,3-butadien...
Text Solution
|
- The IUPAC name of C(6) H(5) COC1 is :
Text Solution
|
- The number of isomers of C(6)H(14) is:
Text Solution
|
- Statement I: Molecules that are non-superimposable on their mirror ima...
Text Solution
|
- The most stable resonating structure is
Text Solution
|
- The correct statement (s) concerning the structure (E), (F), and (G) i...
Text Solution
|
- The correct statements about the compound given below is :
Text Solution
|
- Hyperconjugation involves overlap of which of the following orbitals?
Text Solution
|
- The correct stability order for the following species is
Text Solution
|
- The IUPAC name of the following compound is :
Text Solution
|
- The alkene that exhibits geometrical isomerism is
Text Solution
|
- The correcct statement(s) about the compound. H(3)C(OHHC-CH=CH-CH)OH)C...
Text Solution
|
- The total number of cyclic structure as well as stereoisomers possib...
Text Solution
|