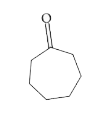
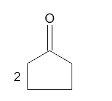
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
VMC MODULES ENGLISH|Exercise Level-2 (Numerical Value Type)|15 VideosHYDROCARBONS
VMC MODULES ENGLISH|Exercise JEE Main Archive|46 VideosHYDROCARBONS
VMC MODULES ENGLISH|Exercise Level-1|75 VideosHALOALKANES AND HALOARENES
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - F|6 VideosICONIC EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-HYDROCARBONS-Level-2
- Statement-1: All the hydrogen atoms in CH(2)=C=CH(2) lie in one plane...
Text Solution
|
- Addition of one equivalent of bromine to 1, 3-pentadiene at temperatur...
Text Solution
|
- Reductive Ozonolysis of will give
Text Solution
|
- H(3)C-underset(CH(3))underset("| ")"CH"-CH-=CH(2)+HBrrarr(X) Here (...
Text Solution
|
- 0.40 g of an organic compound (A), (M.F.-C(5)H(8)O) reacts with x mole...
Text Solution
|
- Which one of the following compounds gives acetone (CH(3))(2)C=O as on...
Text Solution
|
- Which of the following compound was the starting material for the oxid...
Text Solution
|
- Optically active isomer (A) of (C(5)H(9)Cl) on treatment with one mole...
Text Solution
|
- Which alkyne will give 3-ethylhexane on catalytic hydrogenation?
Text Solution
|
- What is the final product, C, of the following reaction sequence ?
Text Solution
|
- Which of the following is aromatic ?
Text Solution
|
- Which of the following species will be aromatic ?
Text Solution
|
- Which of the following is expected to be aromatic ?
Text Solution
|
- C4H6 may contain :
Text Solution
|
- Cyclobutadiene is said to be :
Text Solution
|
- Degree of unsaturation in the following compound is :
Text Solution
|
- The reaction of toluene with chlorine in the presence of ferric chlori...
Text Solution
|
- Name the electrophile produced in the reaction of benzene with benzoyl...
Text Solution
|
- Toluene can be converted into benzaldehyde by oxidation with :
Text Solution
|
- Which of the following is stronger hydrocarbon acid ?
Text Solution
|
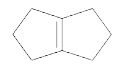 will give
will give