A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-HYDROCARBONS-JEE Advanced Archive
- The compound formed as a result of oxidation of ethyl benzene by KMnO(...
Text Solution
|
- In the following sequence of reactions, the alkene affords the compoun...
Text Solution
|
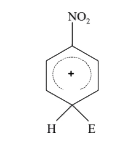
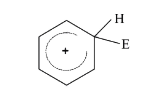
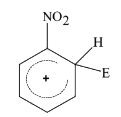
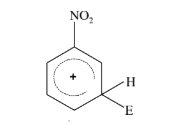
- The electrophile, E^(+) attacks the benzene ring to generate the inter...
Text Solution
|
- The treatment of CH3MgX with CH3C-=C-H produces
Text Solution
|
- The synthesis of 3-octyne is achieved by adding a bromoalkane into a m...
Text Solution
|
- The total number of cyclic isomers possible for a hydrocarbon with the...
Text Solution
|
- One mole of a symmetrical alkene on ozonolysis gives two moles of an a...
Text Solution
|
- The maximum number of isomers (including stereoisomers) that are possi...
Text Solution
|
- Ozonolysis of an organic compound gives formaldehyde as one of product...
Text Solution
|
- Which branched chain isomer of the hydrocarbon with molecular mass 72u...
Text Solution
|
- The number of optically active products obtained from the complete ozo...
Text Solution
|
- Isomers of hexane, based on their branching, can be divided into three...
Text Solution
|
- The major organic compound formed by the reaction of 1, 1, 1– trichlor...
Text Solution
|
- The reactivity of compound Z with different halogens under appropriate...
Text Solution
|
- Compound (S) that on hydrogenation product (S) optically inactive comp...
Text Solution
|
- In the following reaction, the major product is :
Text Solution
|
- What is the major product expected from the following reaction ? Where...
Text Solution
|
- product of above reaction is :
Text Solution
|
- Among the following reactions (s), which gives (give) tert-butyl benze...
Text Solution
|
- The reaction of propene with HOCI (CI(2)+H(2)O) proceeds through the i...
Text Solution
|