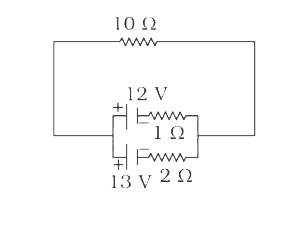
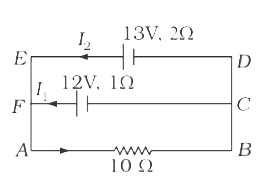
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
DC CIRCUIT
VMC MODULES ENGLISH|Exercise JEE ADVANCED ARCHIVE|68 VideosDC CIRCUIT
VMC MODULES ENGLISH|Exercise LEVEL-2 DAILY TUTORIAL SHEET-5|10 VideosCURRENT ELECTRICITY
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-F|10 VideosDYNAMICS OF A PARTICLE
VMC MODULES ENGLISH|Exercise JEE Advance (Archive) Level - II (SINGLE OPTION CORRECT TYPE )|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-DC CIRCUIT-LEVEL-1 JEE MAIN ARCHIVE
- On interchanging the resistances, the balance point of a meter bridge ...
Text Solution
|
- In a potentiometer experiment it is found that no current passes throu...
Text Solution
|
- Three moles of CO(2) gas expands isothermally (in thermal contact with...
Text Solution
|
- In the given circuit the internal resistance of the 18 V cell is negli...
Text Solution
|
- A corbon resistance has a following colour code. What is the value of ...
Text Solution
|
- Drift speed of electrons, when 1.5 A of current flows in a copper wire...
Text Solution
|
- When the switch S, in the circuit shown, is closed, then the value of ...
Text Solution
|
- AN ideal battery of 4 V and resistance R are connected in series i...
Text Solution
|
- A resistance is shown in the figure. Its value and tolerance are given...
Text Solution
|
- A copper wire is stretched to make it 0.5% longer. The percentage chan...
Text Solution
|
- A current of 2 mA was passed through an unknown resistor which dissipa...
Text Solution
|
- The Wheatstone bridge shown in Fig. here, gets balanced when the carbo...
Text Solution
|
- The actual value of resistance R, shown in the figure is 30 Omega. Thi...
Text Solution
|
- A uniform metallic wire has a resistance of 18 Omega and is bent into ...
Text Solution
|
- In the given circuit the cells have zero internal resistance. The curr...
Text Solution
|
- A potentiometer wire AB having length L and resistance 12 r is joined ...
Text Solution
|
- A 2 W carbon resistor is color coded with green, black, red and brown ...
Text Solution
|
- In the circuit shown, the potential difference between A and B is .
Text Solution
|
- A galvanometer having a resistance of 20 Omega and 30 divisible on ...
Text Solution
|
- In the meter bridge experimental set up, shown in the figure, the null...
Text Solution
|