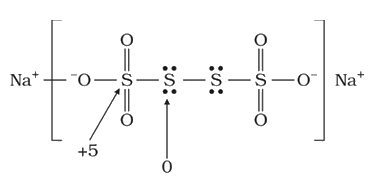
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STOICHIOMETRY-II-JEE Advanced (Archive)
- The value of n in the molecular formula Be(n)Al(2) Si( 6)O(18) is …..
Text Solution
|
- The compound Ba(2)Cu(3)O(7), which shows super conductivity, has coppe...
Text Solution
|
- 5.0 cm^(3) of H(2)O(2) liberates 0.508 g of iodine from an acidified K...
Text Solution
|
- A 20 mL mixture of CO, CH4, and Helium (He) gases is exploded by an el...
Text Solution
|
- A 3.00 g sample containing Fe(3)O(4), Fe(2)O(3) and an inert impure s...
Text Solution
|
- To a 25 mL H(2)O(2) solution excess of an acidified solution of pota...
Text Solution
|
- The number of moles of KMnO(4) that will be needed to react with one ...
Text Solution
|
- An aqueous solution containing 0.5 g KIO3 (formula weight =214.0) was ...
Text Solution
|
- The oxidation number of sulphur in S(8), S(2)F(2) and H(2)S respective...
Text Solution
|
- Amongst the following identify the species with an atom in oxidation...
Text Solution
|
- H(2) O(2) solution (20 mL) reacts quantitatively with a solution of KM...
Text Solution
|
- The 3ClO^(-)(aq.)to ClO(3)^(-)(aq.)+2Cl^(-)(aq.) is an example of
Text Solution
|
- In the standardization of Na(2)S(2)O(3) using K(2)Cr(2)O(7) by iodomet...
Text Solution
|
- The pair of the compounds in which both the metals are in the highest ...
Text Solution
|
- Consider the titration of potassium dichromate solution with acidified...
Text Solution
|
- Match the reactions in column I with nature of the reaction/type of th...
Text Solution
|
- Among the following, what is the number of elements showing only one n...
Text Solution
|
- The difference in the oxidation numbers of two types of sulphul atoms ...
Text Solution
|
- The oxidation states of Cr in [Cr(H(2)O)(6)]Cl(3). ,[Cr(C(6)H(6))(2)...
Text Solution
|
- To measure the quantity of MnCl(2) dissolved in an queous solution, it...
Text Solution
|