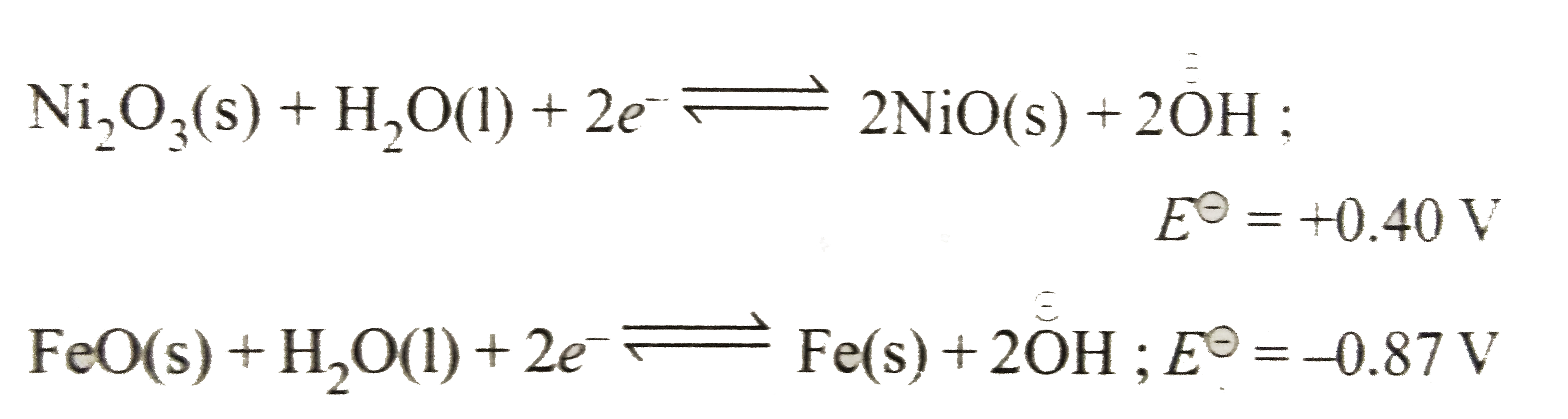Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise FUNDAMENTAL|50 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise ENABLE|50 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|57 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE)|30 VideosENVIRONMENTAL CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|39 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ELECTROCHEMISTRY-JEE Advanced (Archive)
- The standard reduction potential for the half cell : NO(3)^(c-)(aq)+...
Text Solution
|
- Chromium metal is electroplated using an acidic solution containing Cr...
Text Solution
|
- The Edison storage cell is represented as : Fe(s)|FeO(s)|KOH(aq)|Ni(...
Text Solution
|
- The standard reduction potential of the Ag^(o+)|Ag electrode at 298K i...
Text Solution
|
- An excess of liquid mercury is added to an acidicfied solution of 1.0x...
Text Solution
|
- The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate t...
Text Solution
|
- How many grams of silver could be plated out on a serving tray by the ...
Text Solution
|
- Calculate the equilibrium constant for the reaction : Fe^(2+)Ce^(4+)...
Text Solution
|
- Find the solubility product of a saturated solution of Ag(2)CrO(4) in ...
Text Solution
|
- Calculate the equilibrium constant for the reaction, 2Fe^(3+) + 3I^(-)...
Text Solution
|
- The standard reduction potential values of three metallic cations, X, ...
Text Solution
|
- The standard reductino potentials E^(c-) for the half reactinos are as...
Text Solution
|
- The gasX at 1 atm is bubbled through a solution containing a mixture o...
Text Solution
|
- A cell, Ag|Ag^(o+)||Cu^(2+)|Cu , initially contains 1 M Ag^(o+) and 1M...
Text Solution
|
- Copper sulphate solution (250 ML) was electrolyzed using a platinum an...
Text Solution
|
- For the electrochemical cell, (M|M^(o+))||(X^(c-)|X),E^(c-).((M^(o+)|M...
Text Solution
|
- The following electrochemical cell has been set up Pt(1)|Fe^(3+)|Fe^...
Text Solution
|
- A standard solution of KNO(3) is used to make salt bridge, because
Text Solution
|
- The correct order of equivalent conductance at infinite dilution of ...
Text Solution
|
- Standard electrode potential data are useful for understanding the sui...
Text Solution
|