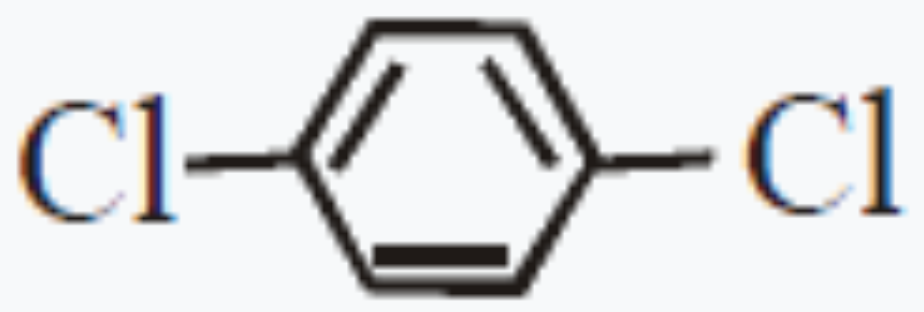
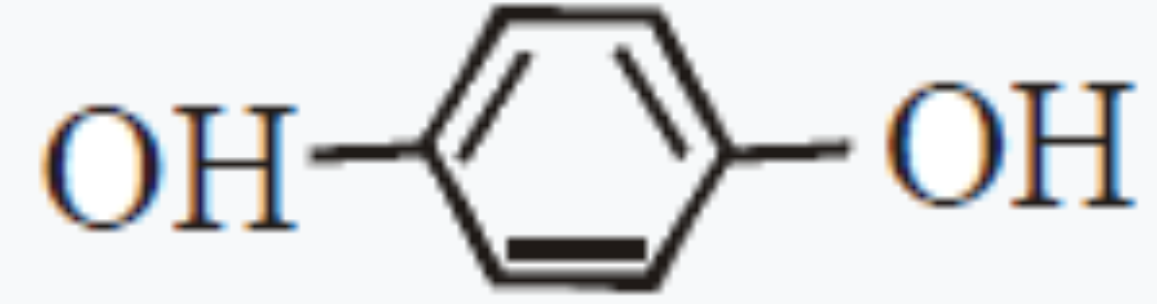
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise EFFICIENT|50 VideosCHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosCHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive )|67 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING & CHEMICAL STRUCTURE -ENABLE
- The molecule which has pyramidal shape is
Text Solution
|
- Which of the following is least ionic ?
Text Solution
|
- Which of the following has a non zero dipole moment ?
Text Solution
|
- The high boiling point of water is due to
Text Solution
|
- The formal charge on P on the phosphonium ion (PH(4)^(+)) is :
Text Solution
|
- Which is not correctly matched ?
Text Solution
|
- Which molecule has the highest boiling point ?
Text Solution
|
- The number of near about 90^@ angles between bond pair - bond pair of ...
Text Solution
|
- The sigma(2s) orbital has a higher energy than sigma(1s)^(**) orbital,...
Text Solution
|
- The atomic numbers of three elements L,M and P are Z,Z+1 and Z+2 respe...
Text Solution
|
- The bonding electrons and lone pairs present in I(3)^(-) are respectiv...
Text Solution
|
- The central atom of a molecule has 33% s-character in its hybrid orbit...
Text Solution
|
- Hydrogen bonding is strongest in
Text Solution
|
- HCI is a gas where/ as HF is a low boiling point liquid because :
Text Solution
|
- The shape of ethylene molecule is
Text Solution
|
- A molecule of chloral hydrate contains two -OH groups attached to a si...
Text Solution
|
- Which one of the following halogens has the highest bond energy ?
Text Solution
|
- If a compound has reasonance (Delta H is usually negative for formatio...
Text Solution
|
- Which one of the following fluorides does not exist in nature ?
Text Solution
|
- A molecule is formed by sp^3d^2 hybridisation. Bond angle in it is :
Text Solution
|