A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosCHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise ENABLE|50 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive )|67 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING & CHEMICAL STRUCTURE -EFFICIENT
- In vinyl acetylene overset(1)( C) H-=overset(2)( C)- overset(3)( C) H=...
Text Solution
|
- The enolic form of acetone (CH(3)-overset(OH)overset(|)C=CH2) contains
Text Solution
|
- Which of the following has the highest percentage of ionic character i...
Text Solution
|
- Which of the following is most polar bond ?
Text Solution
|
- Which of the following molecules has the shortest carbon-carbon bond ?
Text Solution
|
- Which of the following molecule forms linear polymeric structure due...
Text Solution
|
- H2O is dipolar, whereas BeF2 is not. It is because:-
Text Solution
|
- The correct order of dipole moments of HX bond in the given compounds ...
Text Solution
|
- The interatomic distance in H(2) and CI(2) molecules are 74 an d198 pm...
Text Solution
|
- In a diatomic molecule the bond distance is 1 xx 10^(-8) cm. Its dipol...
Text Solution
|
- The hybridisation of carbon in diamond, graphite and acetylene are res...
Text Solution
|
- In OF2, the number of bond pairs and lone pairs of electrons are respe...
Text Solution
|
- Arrange the following species in increasing order of polarizing power ...
Text Solution
|
- The correct order of polarizability for I^(-),Br^(-),CI^(-),F^(-) is :
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: . ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: . ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: . ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: . ...
Text Solution
|
- Ionization energies of five elements in kcal/mol are given below: ...
Text Solution
|
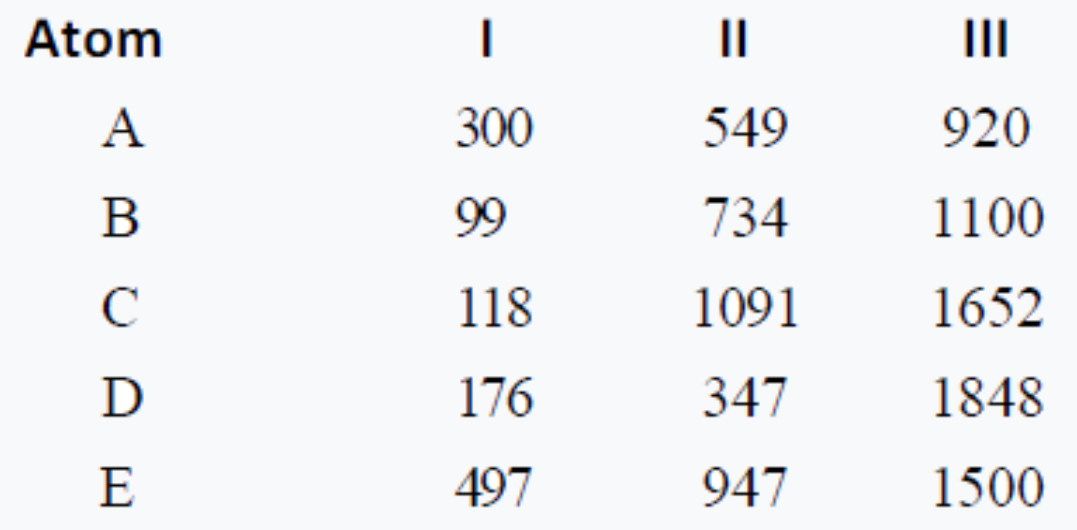 .
.