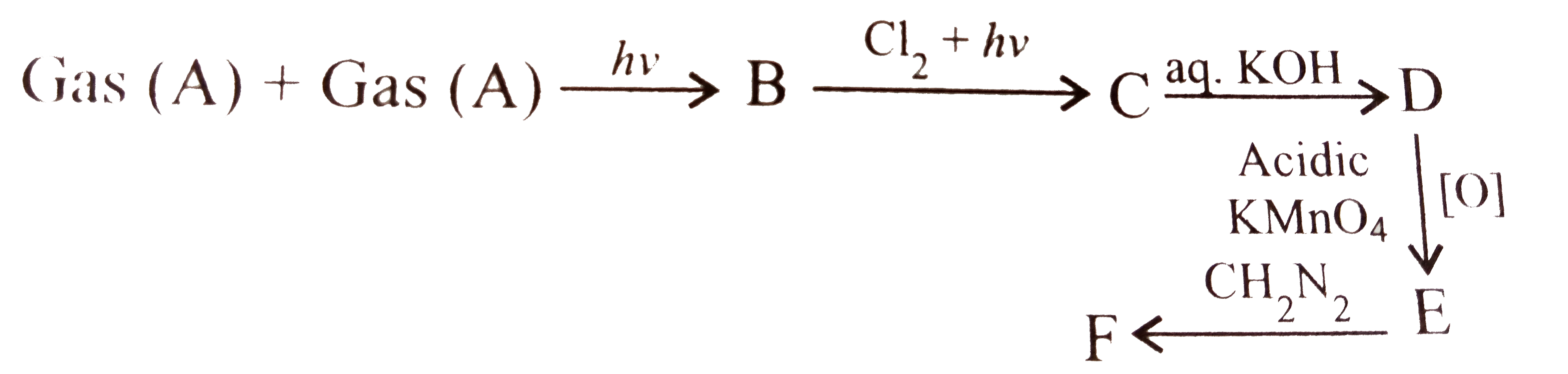A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-EFFICIENT
- Gaseous benzene reacts with hydrogen gas in the presence of nickel cat...
Text Solution
|
- A glass bulb is connected to an open-limb manometer. The level of merc...
Text Solution
|
- For two gases, A and B with molecular weights M(A) and M(B). It is obs...
Text Solution
|
- Certain volume of a gas exerts on its walls some pressure at a particu...
Text Solution
|
- If X(m), X(p) and X(v) represent mole fraction, pressure fraction an...
Text Solution
|
- The root mean square speed of the molecules of a diatomic gas is v. Wh...
Text Solution
|
- At 27^(@) C, hydrogen is leaked through a tiny hole into a vessel for ...
Text Solution
|
- 10 " mL of " a gaseous organic compound containing C, H and O only was...
Text Solution
|
- Five millilitires of a gas (A) containing only C and H was mixed with ...
Text Solution
|
- One mole of nitrogen gas at 0.8 atm takes 38 s to diffuse through a pi...
Text Solution
|
- The average velocity of gas molecules is 400 m/sec calculate its rms v...
Text Solution
|
- A graph is plotted between PV(m) along Y-axis and P along X-axis, wher...
Text Solution
|
- 1.0 litre of N(2) and 7/8 litre of O(2) at the same temperature and pr...
Text Solution
|
- The volumes of two vessels at same temperature are in the ratio of 2 :...
Text Solution
|
- An evacuated glass vessel weighs 50 gm when empty, 148.0 gm filled wit...
Text Solution
|
- A spherical ballon of 21 cm diameter is to be filled with H(2) at NTP ...
Text Solution
|
- At 30^(@)C and 720 mm of Hg, the density of a gas is 1.5 g// l t. Calc...
Text Solution
|
- Density of dry air containing only N2 and O2 is 1.15 g/L at 740 mm of...
Text Solution
|
- A gas bulb of 1 L capacity contains 2.0xx10^(11) molecules of nitrogen...
Text Solution
|
- 8.4 " mL of " gaseous hydrocarbon A was burnt with 50 " mL of " O2 in ...
Text Solution
|