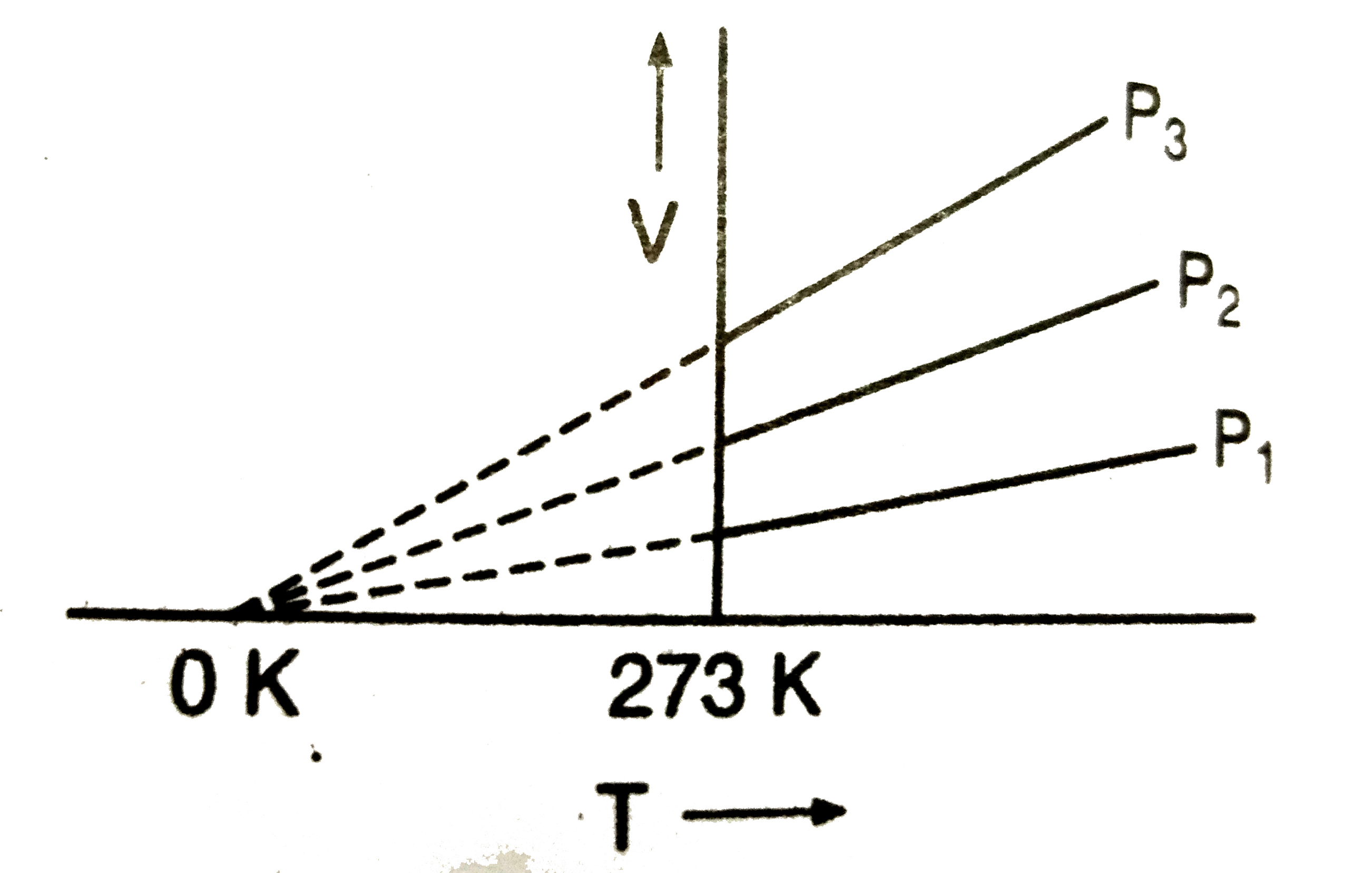
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-IMPECCABLE
- A closed flask contains water in all its three states solid, liquid an...
Text Solution
|
- What is the temperature at which oxygen molecules have the same r.m.s....
Text Solution
|
- The volume-temperature graphs of a given mass of an ideal gas at const...
Text Solution
|
- The most probable velocity of a gas molecule at 298 K is 300 m/s. Its ...
Text Solution
|
- A cylinder of 5 L capacity, filled with air at NTP is connected with a...
Text Solution
|
- Under what conditions will a pure sample of an idela gas not only exhi...
Text Solution
|
- If a gas expands at contant temperature, it indicates that :
Text Solution
|
- The temperature of a gas is raised from 27 ^@ C to 927^@C The root ...
Text Solution
|
- At what temperature, the rate of effusion of N2 would be 1.625 times ...
Text Solution
|
- By the ideal gas law, the pressure of 0.60 mole NH3 gas in a 3.00 L v...
Text Solution
|
- Average K.E. of CO(2) at 27^(@)C is E. the average kinetic energy of N...
Text Solution
|
- At 25^(@)C and 730 mm pressure, 380 mL of dry oxygen was collected.If ...
Text Solution
|
- Volume occupied by one molecule of water (density = 1 g cm^(-3))
Text Solution
|
- At identical temperature and pressure the rate of diffusion of hydroge...
Text Solution
|
- In the temperature changes from 27^(@)C to 127^(@)C, the relative perc...
Text Solution
|
- If a gas expands at contant temperature, it indicates that :
Text Solution
|
- Given: rms velocity of hydrogen at 300K is 1.9 xx 10^3 m/s. The rms ve...
Text Solution
|
- At what temperature, the r.m.s. velocity of a gas measured at 50^(@)C ...
Text Solution
|
- The density of a gas a is twice that of gas B. Molecular mass of A is ...
Text Solution
|
- The compressibility factor for an ideal gas is
Text Solution
|