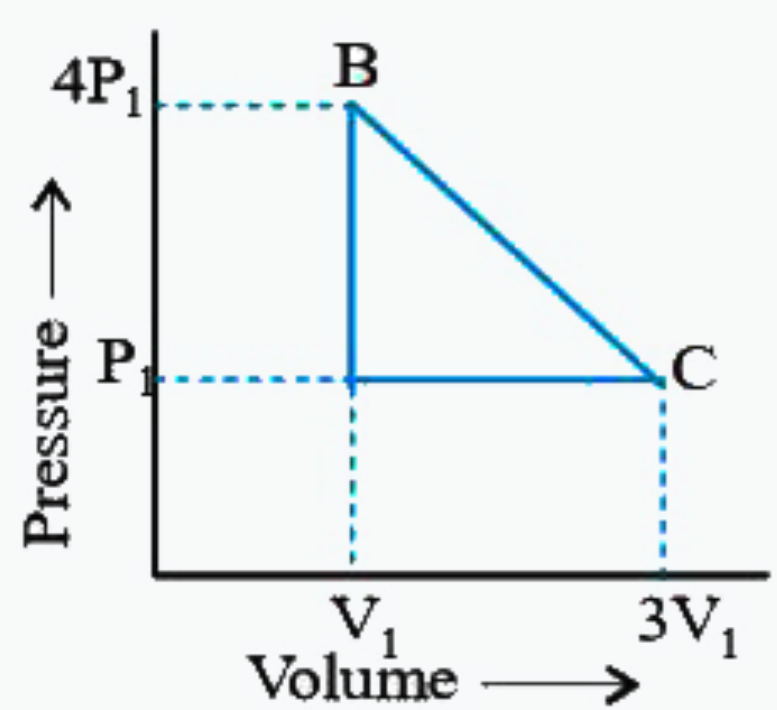
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS & THERMOCHEMISTRY-Efficient
- Consider the following two reactions : (i) "Propene "+H(2) rarr "Pro...
Text Solution
|
- Match the thermodynamic property given in column I with its correct cx...
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown below: Work do...
Text Solution
|
- Which among the following is not an exact differential?
Text Solution
|
- A gas expands adiabatically at constant pressure such that: T prop(1...
Text Solution
|
- In the Haber 's process of ammonia manufacture: N(2) (g)+3H(2) (g) t...
Text Solution
|
- In C(2)H(4) energies of formation of (C=C) and (C-C) are -145 kJ/mol a...
Text Solution
|
- If 150 kJ of energy is needed for muscular work to walk a distance of ...
Text Solution
|
- A mol of Al(3)C(4)(s) reacts with water in a closed vessel at 27^(@)C ...
Text Solution
|
- triangleG^@ and triangleH^(@) for a reaction at 300K are -66.9 kJ/mole...
Text Solution
|
- A gaseous reaction was carried out first keeping the volume constant a...
Text Solution
|
- The standard entropies of CO(2(g)), C((s)), and O(2(g)) are 213.5, 5.7...
Text Solution
|
- Calcualte DeltaH(f)^(@) for chloride ion from the following data: 1/...
Text Solution
|
- Mg(s) + 2HCl(aq) rarr Mg Cl(2) (aq) + H(2)(g) , Delta(r )H^(@) = -467 ...
Text Solution
|
- Given the experimental information below: 2Sr(s) + O(2)(g) rarr 2SrO...
Text Solution
|
- What is the heat of reaction for the following reaction? CH(4)(g)+NH...
Text Solution
|
- 25.0 mL of 1.0M HCl is combined with 35.0 mL of 0.5 M NaOH. The initia...
Text Solution
|
- The reaction 3O(2)(g) rarr 2O(3)(g), Delta(r )H > 0. What can be conc...
Text Solution
|
- When 5.0 mL of a 1.0 M HCl solution is mixed with 5.0 mL of a 0.1 M Na...
Text Solution
|
- Which of the following reactions doesn’t represent the standard state ...
Text Solution
|