A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-LIQUID SOLUTION -EFFICIENT
- Elevation in boiling point of a solution of non-electrolyte in CCl(4) ...
Text Solution
|
- A solution of a non-volatile solute in water has a boiling point of 37...
Text Solution
|
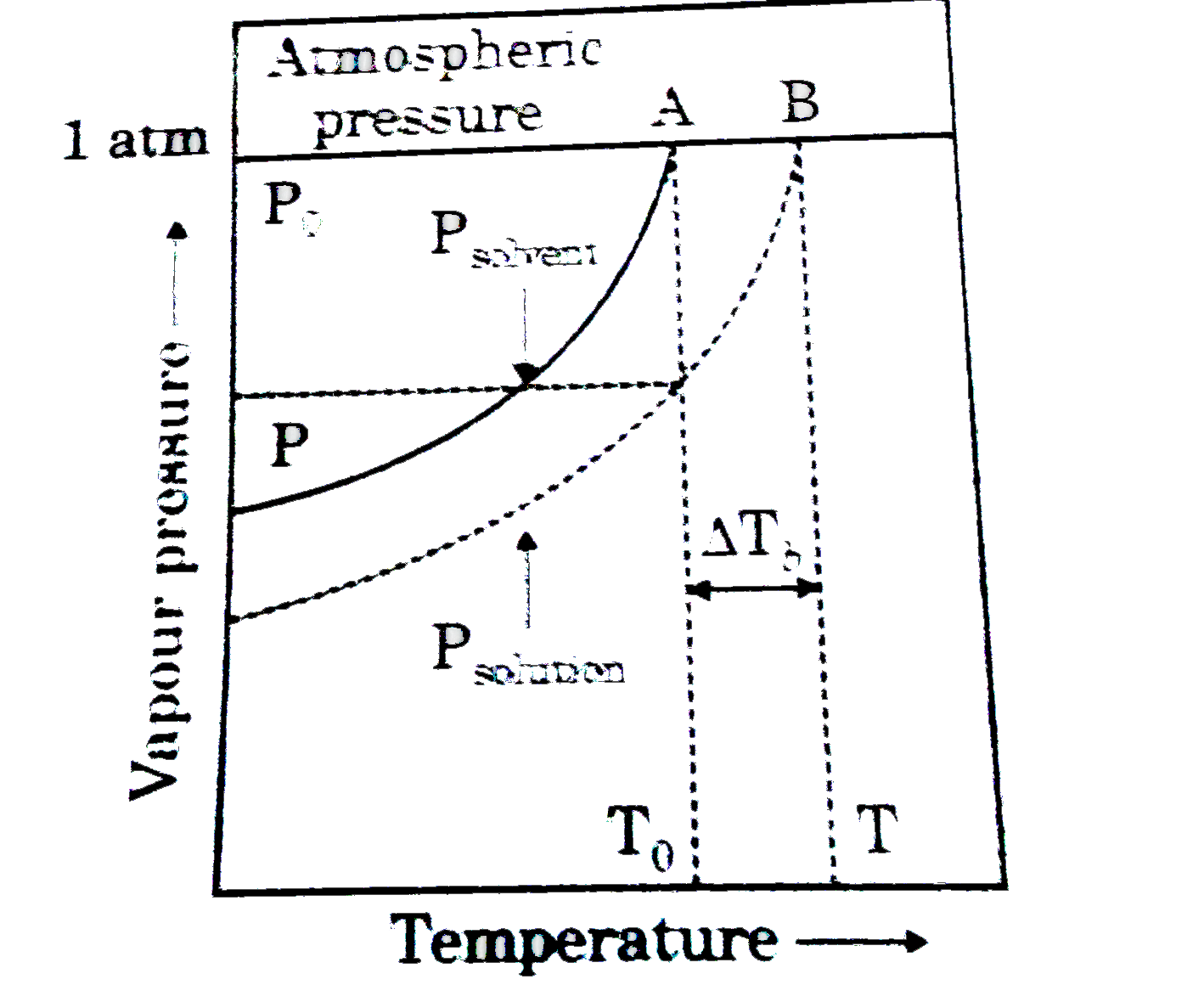
- Figure explains elevation in boiling point when a non-volatile solute ...
Text Solution
|
- Density of 1M solution of a non-electrolyte C(6)H(12)O(6) is 1.18 g/mL...
Text Solution
|
- When a solution w g of urea in 1Kg of water is cooled to -0.372^(@)C,2...
Text Solution
|
- The relative decrease in VP of an aqueous glucose dilute solution is f...
Text Solution
|
- Glucose is added to 1 litre water to such an extent that (DeltaT(f))/(...
Text Solution
|
- A solution containing 4 g of a non-volatile organic solute per 100 mL ...
Text Solution
|
- The osmotic pressure of a decimolar solution of glucose at 30^(@)C is ...
Text Solution
|
- A solution containing 8.6g per dm^(3) of urea (mol. wt. 60) was found...
Text Solution
|
- The osmotic pressure of a solution containing 100 ml of 3.4% solution ...
Text Solution
|
- Two solutions each in 100 ml have 4 g glucose and 10 g sucrose respect...
Text Solution
|
- 5 g of a polymer of molecular mass 50kg mol^(-1) is dissolved in 1dm^(...
Text Solution
|
- 100 mL aqueous solution of glucose with osmotic pressure 1.2 atm at 25...
Text Solution
|
- 10 g of solute A and 20gm of solute B are both dissolved in 500ml wate...
Text Solution
|
- At 10^(@)C, the osmotic pressure of urea solution is 500 mm.The soluti...
Text Solution
|
- The relationship between osmotic pressure (P) at 273 K when 10g glucos...
Text Solution
|
- The vapour pressure of ether at 20^(@)C is 442 mm. When 7.2 g of a sol...
Text Solution
|
- The vapour pressure of pure benzene is 639.7 mm Hg and the vapour pres...
Text Solution
|
- 1 g of monobasic acid in 100 g of water lowers the freezing point by 0...
Text Solution
|