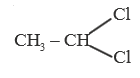A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES & HALOARENES
VMC MODULES ENGLISH|Exercise IMPECCABLE|100 VideosHALOALKANES & HALOARENES
VMC MODULES ENGLISH|Exercise ENABLE|99 VideosENVIRONMENTAL CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|39 VideosHALOALKANES AND HALOARENES
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - F|6 Videos
VMC MODULES ENGLISH-HALOALKANES & HALOARENES -EFFICIENT
- Correct IUPAC name of alkane obtained in the reaciton of 2-chloropropa...
Text Solution
|
- What is the product formed in the following reaction? C(6)H(5)OH + "...
Text Solution
|
- H-C equiv C - H + 2HCl rarrFinal product, final product will be:
Text Solution
|
- 4. Which one of the following compounds does not given C(2)H(5)Cl by t...
Text Solution
|
- Which one of the following compounds will not given iodoform by the re...
Text Solution
|
- Ethyl alcohol gives ethyl chloride with the help of
Text Solution
|
- Hydrolysis of trichloromethane with aqueous KOH gives
Text Solution
|
- If 1,3- dibromopropane reacts with zinc and NaI, the product obtained ...
Text Solution
|
- The compound obtained by heating a mixture of a primary amine and chlo...
Text Solution
|
- product A is:
Text Solution
|
- The reaction of ethylene bromide with zinc yields:
Text Solution
|
- Phosgene is:
Text Solution
|
- Identify (Z) in the following reaction series, C2H5Ioverset("Alcoho...
Text Solution
|
- A and B in the following reactions are respectively (i) CHCl(3) over...
Text Solution
|
- Ethyl bromide reacts with sodium lead alloy to form
Text Solution
|
- Which alkyl chloride shows highest probability of SN1 mechanism?
Text Solution
|
- C(6)H(6) + CH(3) Cl underset(AlCl(3))overset("Anhydrous")(rarr) C(6)H(...
Text Solution
|
- The product obtained by the reaction of silver propanoate with bromine...
Text Solution
|
- Which one of the following gives iodoform test?
Text Solution
|
- Chloroform is:
Text Solution
|