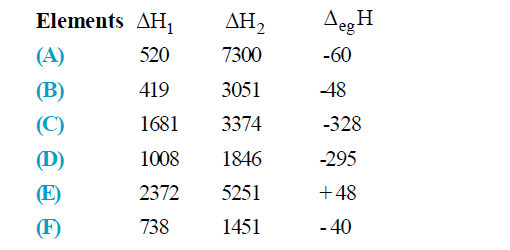A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-PERIODIC PROPERTIES OF ELEMENTS-EFFICIENT
- Predict the blocks and periods of the following elements. O (8)
Text Solution
|
- Predict the blocks and periods of the following elements. Pb (82)
Text Solution
|
- The first and second ionization enthalpies and electron gain enthalpy ...
Text Solution
|
- Predict the blocks and periods of the following elements. Se (34)
Text Solution
|
- Predict the blocks and periods of the following elements. Sn (50)
Text Solution
|
- The element with the electronic configuration [Xe]^(54) 4f^(14)5d^(1)6...
Text Solution
|
- Which among the following is not noble gas?
Text Solution
|
- With which of the following electronic configuration of an atom has th...
Text Solution
|
- Identify the correct order of the size of the following .
Text Solution
|
- Which of these salts shows the least solubility in water?
Text Solution
|
- Which among the following is not noble gas?
Text Solution
|
- Which has the highest melting point?
Text Solution
|
- Because of lanthanoid contraction, which of the following pairs of el...
Text Solution
|
- Noble gases belong to which group of periodic table.
Text Solution
|
- Ion channels have been discovered by
Text Solution
|
- The helical form of the periodic table was given by De Chancourtouis a...
Text Solution
|
- The most basic oxide among the following is
Text Solution
|
- Which of the following is a neutral oxide?
Text Solution
|
- In which element the electrons will be experiencing the highest effect...
Text Solution
|
- Among which of these element pairs is the increase in radius minimum?
Text Solution
|