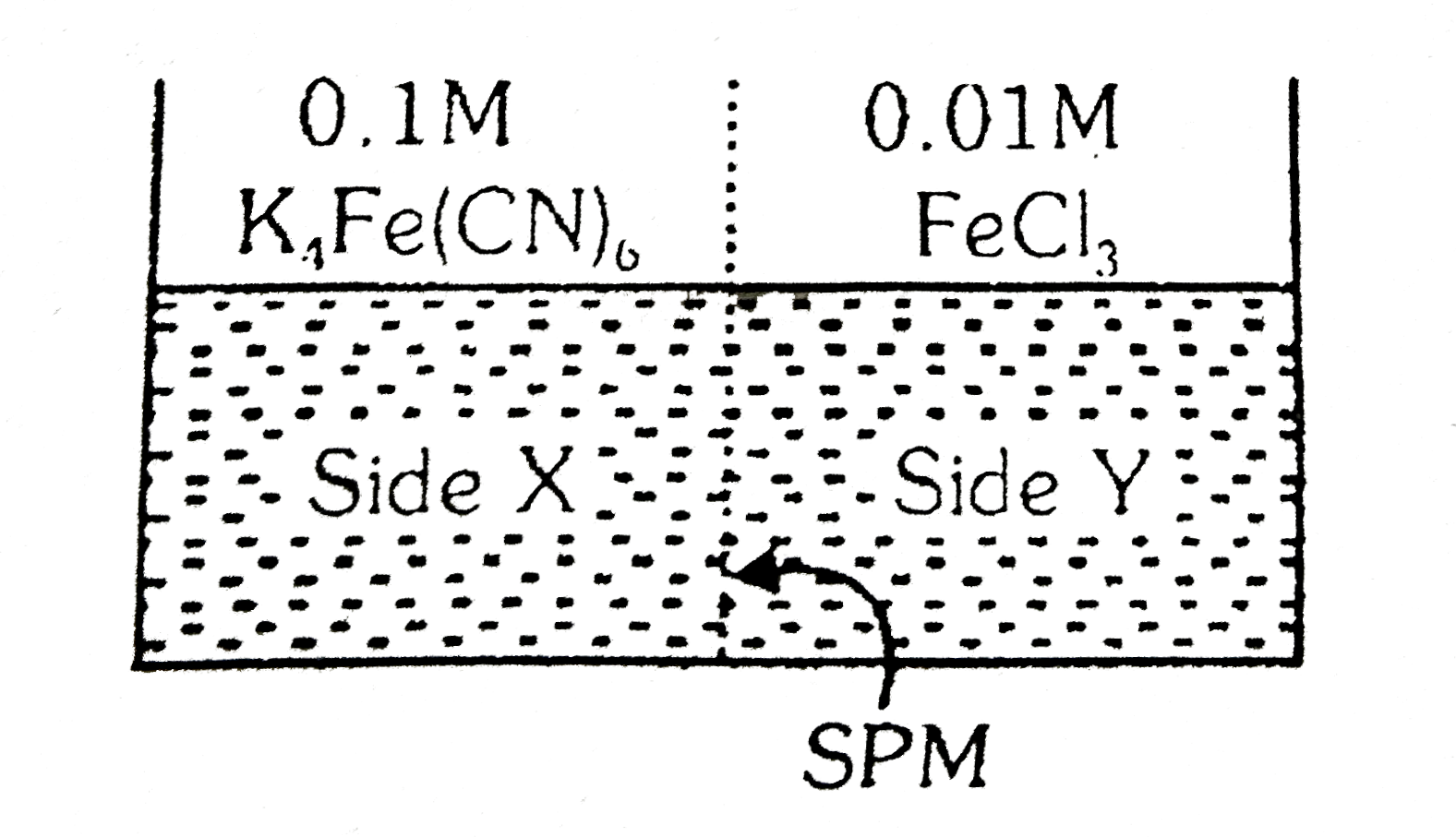A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-LIQUID SOLUTION -IMPECCABLE
- FeCI(3) on reaction with K(4)[Fe(CN)(6)] in aqueous solution gives blu...
Text Solution
|
- What is the mole fraction of the solute in a 1.00 m aqueous solution ?
Text Solution
|
- Which of the following electrolytes has the same value of van't Hoff f...
Text Solution
|
- The boiling point of 0.2 mol kg^(-1) solution of X in water is greater...
Text Solution
|
- Of the following 0.10 m aqueous solutions, which one will exhibit the ...
Text Solution
|
- How many grams of concentrated nitric acid solution should be used to ...
Text Solution
|
- If excess of AgNO(3) solution is added to 100 mL of a 0.024 M solution...
Text Solution
|
- 6.02 xx 10^(20) molecules of urea are present in 100 mL of its solutio...
Text Solution
|
- p(A) and p(B) are the vapour pressure of pure liquid components A and ...
Text Solution
|
- The vapour pressure of chloroform (CHCl)(3) and dichlorocethene (CH(2)...
Text Solution
|
- 58.5 g of NaCl and 180 g of glucose were separately dissolved in 1000 ...
Text Solution
|
- Which of the following shows negative deviation from Raoult's law?
Text Solution
|
- The mass of a non-volatile solute of molar mass 40g" "mol^(-1) that sh...
Text Solution
|
- The solution which has higher osmotic pressure than some other solutio...
Text Solution
|
- The vapour pressure lowering caused by addition of 100 g of sucrose (m...
Text Solution
|
- Molarity of a given orthophosphoric acid solution is 3M. Its normality...
Text Solution
|
- 138 g of ethyl alcohol is mixed with 72 g of water. The ratio of mole ...
Text Solution
|
- In which of these solutions Raoult’s law is not applicable?
Text Solution
|
- The freezing point of 1 m NaCl solution assuming NaCl to be 100% disso...
Text Solution
|
- A solution of two liquids boils at a temperature more than the boiling...
Text Solution
|
- Choose the correct statement: When concentration of a salt solution ...
Text Solution
|