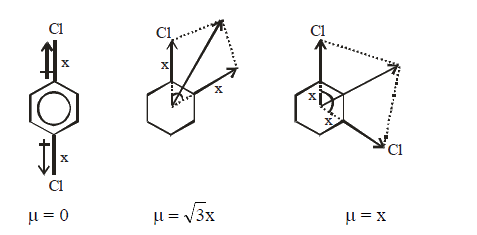
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|36 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-A|10 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL BONDING & CHEMICAL STRUCTURE
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING & MOLECULAR STRUCTURE -SOLVED EXAMPLES
- Which of the following substance has the highest melting point? .
Text Solution
|
- Zeise's salt contains which type of bonds ?
Text Solution
|
- Which is distilled first ?
Text Solution
|
- Which factor is most responsible for the increase in boiling points of...
Text Solution
|
- Which of the following resonating structures is not correct for CO(2) ...
Text Solution
|
- Which of the following does not contain any coordinate bond ?
Text Solution
|
- In which of the following species the angle around the central atom is...
Text Solution
|
- Arrange the following in decreasing order of dipole moment (1) m-Dich...
Text Solution
|
- In which of the following diatomjic molecules/ions is the bond order o...
Text Solution
|
- Bond order of N-O bonds in nitrate ion is
Text Solution
|
- Which of the following is paramagnetic ?
Text Solution
|
- Among LiCl, RbCl, BeCl2, MgCl2, the compounds with greatest and least ...
Text Solution
|
- Which of the following molecular orbitals has two nodal planes ?
Text Solution
|
- In PO(4)^(3-) the formal charge on each O-atom and P-O bond order resp...
Text Solution
|
- Among the following ions the ppi-dpi overlap could be present in
Text Solution
|
- Which of the following molecules is linear ?
Text Solution
|
- Which of the following hydride is ionic ?
Text Solution
|
- A covalent molecole AB(4) (not a complex) will have which of the follo...
Text Solution
|
- How many type of bond lengths are there in SO(3) ^(2-) ?
Text Solution
|
- A molecule XY2 contains two sigma, two pi and one lone pair of electro...
Text Solution
|