Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
VMC MODULES ENGLISH|Exercise SOLVED EXAMPLES|20 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE-1|3 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise JEE-Advanced|78 VideosSTATES OF MATTER
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosSTOICHIOMETRY - I
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STATES OF MATTER-ILLUSTRATION
- What is the volume of 6 g of hydrogen at 1.5 atm and 273^(@)C ?
Text Solution
|
- Calculate the volume occupied by 7 g of nitrogen gas at 27^(@)C and 75...
Text Solution
|
- Calculate the temperature of 2.0 moles of a gas occupying a volume of ...
Text Solution
|
- The density of ammonia at 30^(@)C and 5 atm pressure is
Text Solution
|
- Pressure of 1 g of an ideal gas A at 27 ^(@)C is found to be 2 bar. Wh...
Text Solution
|
- 2 g of a gas collected over water at 20^(@)C and under a pressure of 7...
Text Solution
|
- If 250ml of N(2) at 30^(@)C and a pressure of 250mm Hg are mixed with ...
Text Solution
|
- 20 dm^(3) of SO(2) diffuse through a porous partition in 60 s. what vo...
Text Solution
|
- Through the two ends of a glass tube of length 200cm hydrogen chloride...
Text Solution
|
- From two identical holes, nitrogen and an unknown gas are leaked into...
Text Solution
|
- The pressure in a vessel that contained pure oxygen dropped from 2000 ...
Text Solution
|
- One mole of He gas at 0.6 atm takes 40 s to diffuse through a pin hole...
Text Solution
|
- 8.4 " mL of " gaseous hydrocarbon A was burnt with 50 " mL of " O2 in ...
Text Solution
|
- Calculate the root mean square, average and most probable speeds of ox...
Text Solution
|
- At a certain temperature 6% molecules of a gas have speed 2 m//s, 9% h...
Text Solution
|
- Calculate the pressure exerted by 10^(23) gas particles each of mass 1...
Text Solution
|
- A gas container of 1.5 dm^(3) capacity contains 3.011 × 10^(23) molecu...
Text Solution
|
- Calculate the total and average kinetic energy of 32 g methane molecul...
Text Solution
|
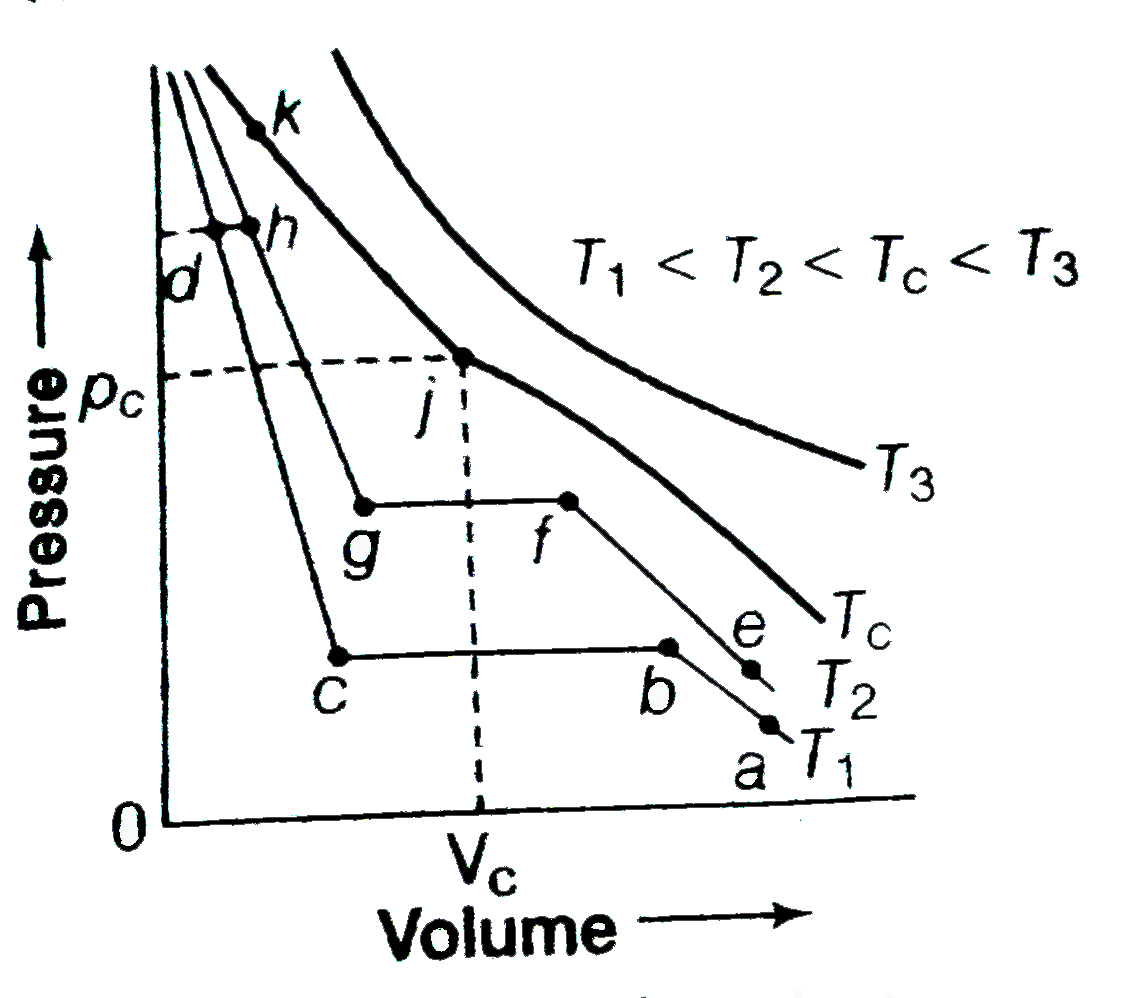
- Isotherms of carbon dioxide at various temperatures are repersented in...
Text Solution
|
- The kinetic molecular theory attributes an average kinetic theory of (...
Text Solution
|