Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
VMC MODULES ENGLISH|Exercise SOLVED EXAMPLES|19 VideosCHEMICAL THERMODYNAMICS
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|22 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE)|30 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL THERMODYNAMICS -IN - CHAPTER EXERCISE - L
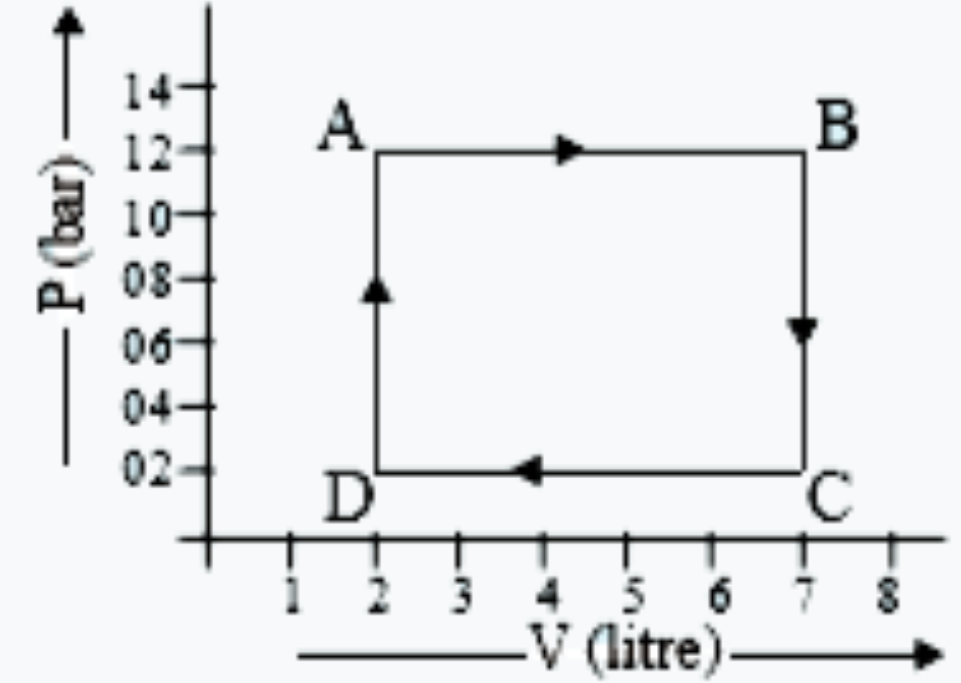
- The diagram shows a P - V graph of a thermodynamic behaviour of an ide...
Text Solution
|
- The bond dissociation energies of X2, Y2 and XY are in the ratio of 1 ...
Text Solution
|
- Energy required to dissociate 4 g of gaseous hydrogen into free gaseou...
Text Solution
|
- The bond dissociation energy needed to form the benzyl radical from to...
Text Solution
|
- Which of the following bonds has the highest energy?
Text Solution
|
- Bond dissociation energies of H(2),Cl(2) and HCl((g)) are 104, 58 and ...
Text Solution
|
- If the first ionization enthalpy of Li is 5.4 eV and the elec- tron ga...
Text Solution
|
- Given that C(g)+4H(g)to CH(4)(g) , Delta H =-166 kJ. The bond energy o...
Text Solution
|
- The H – H bond energy is 430 kJ mol^(-1) and Cl - C bond energy is 240...
Text Solution
|
- If enthalpies of methane and ethane are respectively 320 and 360 calor...
Text Solution
|
- If the bond energies of H-H, Br - Br and H - Br and 433, 192 and 364 m...
Text Solution
|