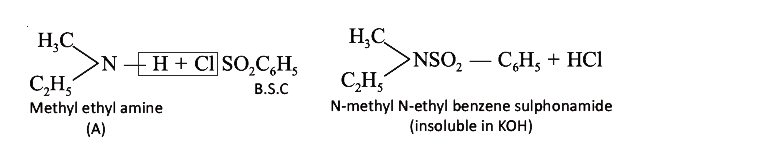Text Solution
Verified by Experts
Topper's Solved these Questions
AMINES
VMC MODULES ENGLISH|Exercise SOLVED EXAMPLES|21 VideosAMINES
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE:|23 VideosAMINES
VMC MODULES ENGLISH|Exercise IMPECCABLE|48 VideosALDEHYDES,KETONES & CARBOXYLIC ACIDS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-M|10 VideosATOMIC STRUCTURE
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive )|67 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-AMINES-ILLUSTRATION
- Give the structure of (A) (explanations are not required). A (C3H9N) r...
Text Solution
|
- Complete the following with appropriate structures : 2,3 "-Dinitroan...
Text Solution
|
- Do the following conversions. Cyclopentene to Chclopentyl amine
Text Solution
|
- Do the following conversions. Acetic acid to glycine (alpha- amino a...
Text Solution
|
- Do the following conversions. Ethyl cyanide to ethylamine
Text Solution
|
- Do the following conversions. Succinic acid to 3-aminopropanoic acid
Text Solution
|
- How will you bring about the following conversions ? (i) Benzoic aci...
Text Solution
|
- Write the structure of the foul-smelling compound obtained when anilin...
Text Solution
|
- Give reason for the following in one or two sectences : 'Dimethyl a...
Text Solution
|
- The product og trsvyion : CH(3) CH(2) OH+ Cu overset(300^(@) C) to ...
Text Solution
|
- Conversion of Nitrobenzene to m-bromochlorobenzene
Text Solution
|
- Accomplish the following conversions : Chlorobenzene to p-bromoanilin...
Text Solution
|
- Do the following conversions: Aniline to benzoic acid
Text Solution
|
- How will you bring about the following conversion ? 4-nitroaniline ...
Text Solution
|
- Do the following conversions: o-cresol to salicyclic acid
Text Solution
|
- A and B respectively are:
Text Solution
|
- Identity A,B,C……………(A,B…………. are all organic compound)
Text Solution
|
- Identity A,B,C……………(A,B…………. are all organic compound)
Text Solution
|
- Identity A,B,C……………(A,B…………. are all organic compound)
Text Solution
|
- Identity A,B,C……………(A,B…………. are all organic compound)
Text Solution
|