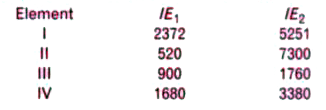Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC PROPERTIES OF ELEMENTS
VMC MODULES ENGLISH|Exercise Practice Exercise|29 VideosPERIODIC PROPERTIES OF ELEMENTS
VMC MODULES ENGLISH|Exercise In Chapter Exercise-A|20 VideosPERIODIC PROPERTIES OF ELEMENTS
VMC MODULES ENGLISH|Exercise In Chapter Exercise-F|20 VideosQUIZ
VMC MODULES ENGLISH|Exercise Chemistry|15 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-PERIODIC PROPERTIES OF ELEMENTS -Solved Examples
- The first ionization enthalpy of magnesium is higher than that of sodi...
Text Solution
|
- Arrange the following elements in the increasing order of non metallic...
Text Solution
|
- The first (IE(1)) and the second (IE(2)) ionisation energies (kJ mol^(...
Text Solution
|
- The first (IE(1)) and the second (IE(2)) ionisation energies (kJ mol^(...
Text Solution
|
- The first (IE(1)) and the second (IE(2)) ionisation energies (kJ mol^(...
Text Solution
|
- The first(IE(1)) and second (IE(2))Ionization energies (KJ/mol) of a f...
Text Solution
|
- Ionic radii of (1) Ti^(4+) lt Mn^(7+) (2) ""^(35)Cl^(-) lt ""^(37)...
Text Solution
|
- Which one of the following indicates the correct order of atomic size?...
Text Solution
|
- If Aufbau rule is not followed, K-19 will be placed in ….. Block (1)...
Text Solution
|
- Period to which cadmium belongs is
Text Solution
|
- An element M has an atomic number 9 and mass number 19. Its ion will...
Text Solution
|
- ""^(238)U(IIIB) changes to ""^(234)Th by emission of alpha-particle. ...
Text Solution
|
- Which of the following transitions involves maximum energy? (1) M^(-...
Text Solution
|
- The process requiring absorption of energy is (1) F to F^(-) (2) Cl ...
Text Solution
|
- The elecron affinity of chlorine is 3. 7 eV. How much energy in kcal i...
Text Solution
|
- Answer the following question (Based on EA, Delta(eg)H^(ɵ) and IE). I...
Text Solution
|
- The ground state electron configurations listed here are incorrect. Id...
Text Solution
|
- The ground state electron configurations listed here are incorrect. Id...
Text Solution
|
- The ground state electron configurations listed here are incorrect. Id...
Text Solution
|
- Draw orbital diagrams for atoms with the following electronic configur...
Text Solution
|