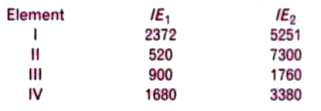Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC PROPERTIES OF ELEMENTS
VMC MODULES ENGLISH|Exercise Practice Exercise|29 VideosPERIODIC PROPERTIES OF ELEMENTS
VMC MODULES ENGLISH|Exercise In Chapter Exercise-A|20 VideosPERIODIC PROPERTIES OF ELEMENTS
VMC MODULES ENGLISH|Exercise In Chapter Exercise-F|20 VideosQUIZ
VMC MODULES ENGLISH|Exercise Chemistry|15 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-PERIODIC PROPERTIES OF ELEMENTS -Solved Examples
- Write balanced equations for the reactions between each of the followi...
Text Solution
|
- Write balanced equations for the reactions between each of the followi...
Text Solution
|
- Write balanced equations for the reactions between each of the followi...
Text Solution
|
- Arrange the elements in each of the following groups in increasing ord...
Text Solution
|
- Arrange the elements in each of the following groups in increasing ord...
Text Solution
|
- Two atoms have the electron configurations 1s^2 2s^2 2p^6 and 1s^2 2s^...
Text Solution
|
- Neon (10) belongs to which group and period of periodic table.
Text Solution
|
- Account for the decrease in first ionization energy between phosphorou...
Text Solution
|
- Among the elements of the third period (Na to Ar), pick out the elemen...
Text Solution
|
- Among the elements of the third period (Na to Ar), pick out the elemen...
Text Solution
|
- Among the elements of the third period (Na to Ar), pick out the elemen...
Text Solution
|
- Among the elements of the third period (Na to Ar), pick out the elemen...
Text Solution
|
- Of the two elements given in each of the following cases, choose the e...
Text Solution
|
- Of the two elements given in each of the following cases, choose the e...
Text Solution
|
- The first ionization enthalpy of magnesium is higher than that of sodi...
Text Solution
|
- Arrange the following elements in the increasing order of non metallic...
Text Solution
|
- The first (IE(1)) and the second (IE(2)) ionisation energies (kJ mol^(...
Text Solution
|
- The first (IE(1)) and the second (IE(2)) ionisation energies (kJ mol^(...
Text Solution
|
- The first (IE(1)) and the second (IE(2)) ionisation energies (kJ mol^(...
Text Solution
|
- The first(IE(1)) and second (IE(2))Ionization energies (KJ/mol) of a f...
Text Solution
|