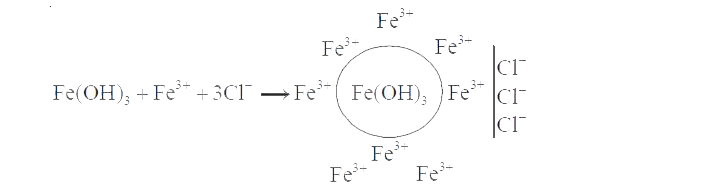A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
SURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise IN-CHAPTER-A|10 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise IN-CHAPTER-B|8 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise ILLUSTRATIONS|14 VideosSTRUCTURE OF ATOM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-F|7 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise EXERCISE-J|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-SURFACE CHEMISTRY-SOLVED EXAMPLES
- Rate of physisorption increases with
Text Solution
|
- Lyophilic sols are:
Text Solution
|
- A white precipitate of Sn(OH)4 is peptized with dil. HCl. The sol part...
Text Solution
|
- Which of the following reactions leads to the formation of a substance...
Text Solution
|
- Graph between log x/ m and log P is a straight line inclined at an ang...
Text Solution
|
- For adsorption, the thermodynamic requirement is that
Text Solution
|
- 1 mol of [Agl]I^(-) can be coagulated by:
Text Solution
|
- The process of passing of a precipitate into colloidal solution on add...
Text Solution
|
- The Brownian movement of colloidal particles is because of:
Text Solution
|
- The stabillization of the dispersed phase in a lyopobic sol is due to
Text Solution
|
- When FeCl3 solution is added to NaOH a negatively charged sol is obta...
Text Solution
|
- Milk is an example of:
Text Solution
|
- Which among the following process is used to remove grease from soap
Text Solution
|
- Which of the following forms cationic micelles above certain concentra...
Text Solution
|
- Which among the following gas will be adsorbed most readily by activat...
Text Solution
|