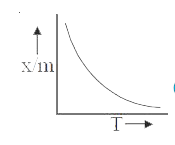
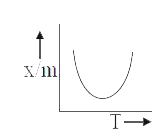
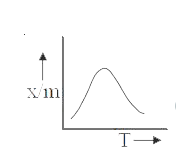
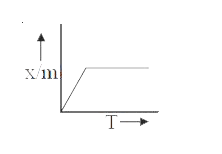
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise IN CHAPTER-C|6 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise IN CHAPTER-D|9 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise IN-CHAPTER-A|10 VideosSTRUCTURE OF ATOM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-F|7 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise EXERCISE-J|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-SURFACE CHEMISTRY-IN-CHAPTER-B
- For the adsorption of a gas on a solid, the plot of log(x/m) versus lo...
Text Solution
|
- According to Langmuir adsorption isotherm, the amount of gas adsorbed ...
Text Solution
|
- Which plot is the adsorption isobar for chemisorption?
Text Solution
|
- If x is amount of adsorbate and ‘m’ is amount of adsorbent, which of t...
Text Solution
|
- According to Freundlich adsorption isotherm, which of the following is...
Text Solution
|
- In freundlich adsorption isotherm, the value of 1//n is
Text Solution
|
- For the adsorption of a gas on a solid, the plot of log(x/m) versus lo...
Text Solution
|
- The curve showing the variation of pressure with temperature for a giv...
Text Solution
|