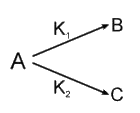
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The substance undergoes first order decomposition. The decomposition f...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- The first order rate constant for the decomposition of N(2)O(5) is 6.2...
Text Solution
|
- A substance undergoes first order decomposition. It follows two first ...
Text Solution
|
- The substance undergoes first order decomposition.The decomposition fo...
Text Solution
|
- A substance '' was found to undergo two parallel first order reaction ...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- A substance undergoes first order decomposition. The decomposition fol...
Text Solution
|
- The substance undergoes first order decomposition. The decomposition f...
Text Solution
|