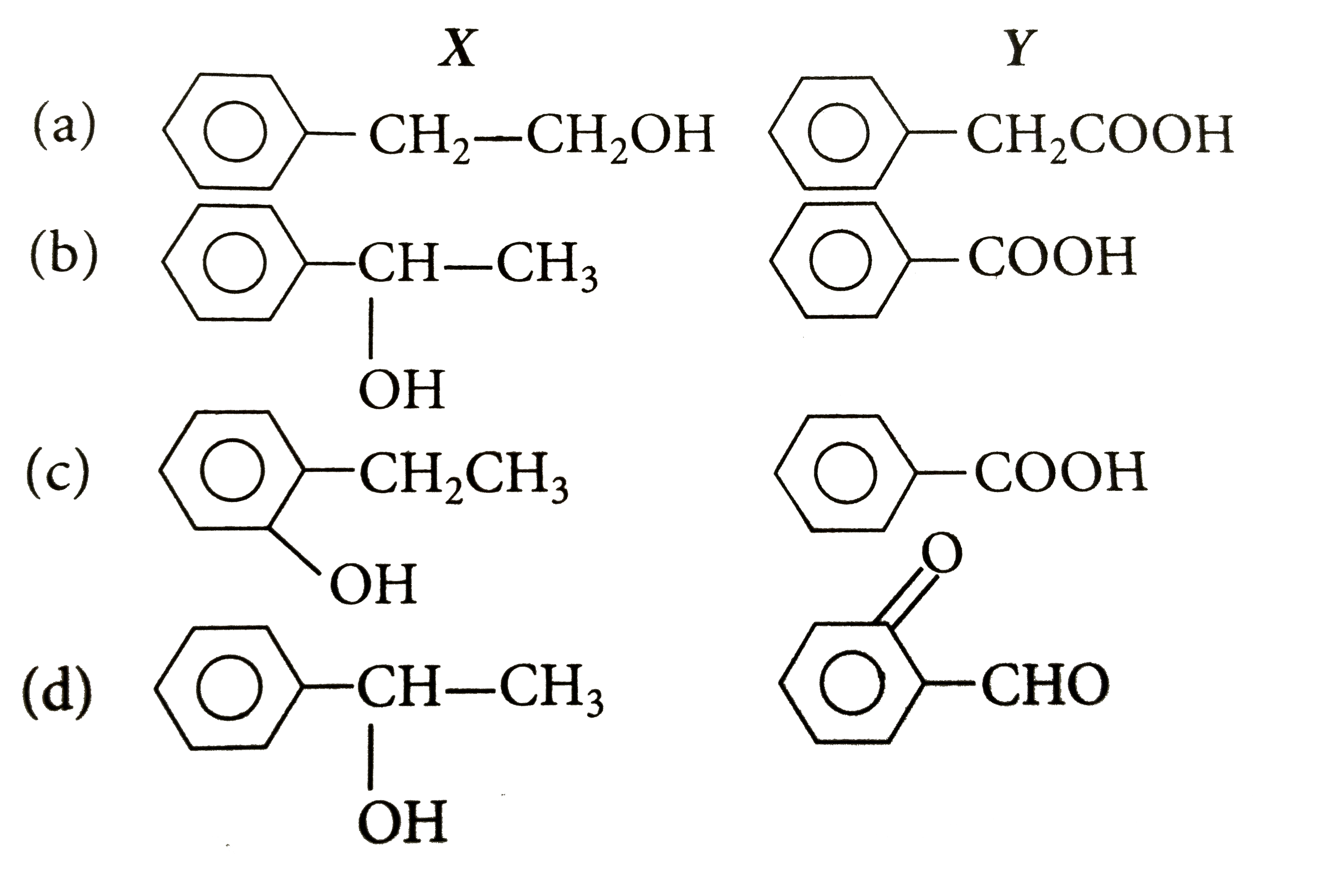
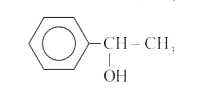
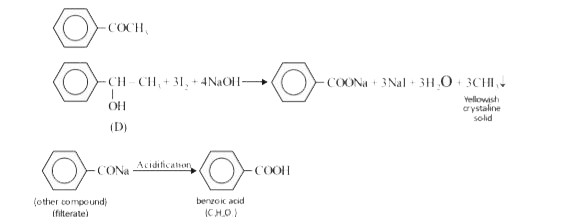
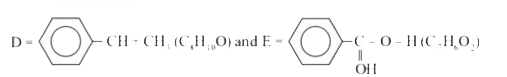Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Example|20 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE:1|21 Videos13-14 HYDROGEN & S-BLOCK ELEMENT
VMC MODULES ENGLISH|Exercise JEE Advanced Archive|48 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Impeccable|100 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ALCOHOLS, PHENOLS & ETHERS -IN-CHAPTER EXERCISE - L
- A compund X(C8H10O) upon treatment with alkaline solution of iodine gi...
Text Solution
|
- 4-chloro-3,5-dimethyl phenol is called :
Text Solution
|
- Alcoholic fermentation is brought about by the action of
Text Solution
|
- Rectified spirit is a mixture of
Text Solution
|
- Methyl alcohol is toxic. The reason assigned is:
Text Solution
|
- Glycerol is used .
Text Solution
|
- Glycerol is not used in
Text Solution
|
- Liquor poisoning is due to:
Text Solution
|
- In order to make alcohol undrinkable pyridine and methanol are added t...
Text Solution
|
- Denatured spirit is mainly used as a :
Text Solution
|
- Main constituent of dynamite is
Text Solution
|