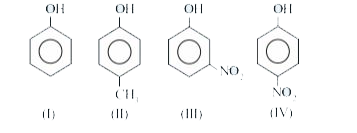
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE:1|21 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE:2|12 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - L|10 Videos13-14 HYDROGEN & S-BLOCK ELEMENT
VMC MODULES ENGLISH|Exercise JEE Advanced Archive|48 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Impeccable|100 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ALCOHOLS, PHENOLS & ETHERS -Example
- Which of the following is soluble in water
Text Solution
|
- Ethyl alcohol is heated with conc. H(2)SO(4) at 170^(@)C. The product ...
Text Solution
|
- The compound that reacts fastest with Lucas reagent at room temperatu...
Text Solution
|
- Diethyl ether on heating with conc. HI gives two moles of:
Text Solution
|
- An industrial method for preparation of methanol is:
Text Solution
|
- Phenol when treated with excess of bromine water gives a white precipi...
Text Solution
|
- In CH(3)CH(2)OH, the bond that undegoes heterolytic clevage most read...
Text Solution
|
- Phenol reacts with bromine in carbon disulphide at low temperature to ...
Text Solution
|
- The products of combustion of an aliphatic thiol (RSH) at 298K are
Text Solution
|
- The order of aciditic strength among the given phenols is :
Text Solution
|
- (CH(3) )(3) CMgBr on reaction with D(2)O produces
Text Solution
|
- Benzenediazonium chloride on reaction with phenol in weakly ba...
Text Solution
|
- Among the following compounds, the strongest acid is :
Text Solution
|
- 1-propanol and 2-propanol can be best distinguished by :
Text Solution
|
- The compound will react most readily with NaOH of form methanol is :
Text Solution
|
Text Solution
|
- The following compound on hydrolysis in aqueous acetone will give :
Text Solution
|
- When phenyl magnesium bromide reacts with t-butanol the product would ...
Text Solution
|
- The best method to prepare cyclohexene form cyclohexanol is by using:
Text Solution
|
- (I) 1,2-Dihydroxy benzene (II) 1,3-Dihydroxy benzene (III) 1,4-Dih...
Text Solution
|