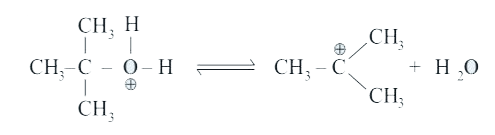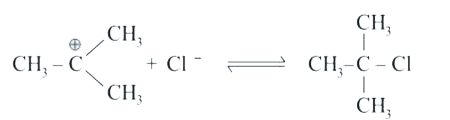A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE:2|12 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE:3|8 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Example|20 Videos13-14 HYDROGEN & S-BLOCK ELEMENT
VMC MODULES ENGLISH|Exercise JEE Advanced Archive|48 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Impeccable|100 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ALCOHOLS, PHENOLS & ETHERS -PRACTICE EXERCISE:1
- Oxo process:
Text Solution
|
- on dehydration with conc. H(2)SO(4) forms predominantly :
Text Solution
|
- In the reaction: CH(3)-underset(CH(3))underset(|)overset(CH(3))overset...
Text Solution
|
- Reduction of gt C = O to gt CH(2) can be carried out with
Text Solution
|
- The boiling points to isomeric alcohols follow the order
Text Solution
|
- The acidic character of 1^(@),2^(@),3^(@) alcohls, H(2)O and RC-=CH i...
Text Solution
|
- In the following reaction sequence : R-OH overset(P+I(2))rarr R-I ov...
Text Solution
|
- Which of the following is the most reactive with HCl in the presence o...
Text Solution
|
- Methanol is manufactured by passing ( under pressure ) a mixture of wa...
Text Solution
|
- CH(3)underset(Br)underset(|)(C)HCH(3)overset("alc."//KOH)toAoverset(HB...
Text Solution
|
- The compound that reacts fastest with Lucas reagent at room temperatu...
Text Solution
|
- A compound that gives a positive iodofrm test is :
Text Solution
|
- One of the cmmercial production methods of methanol is :
Text Solution
|
- HBr reacts fastest with
Text Solution
|
- Which of the following compounds is oxidised to prepare methyl ethyl k...
Text Solution
|
- The order of reactivity of the following alcohols towards conc. HCI is
Text Solution
|
- The product of acid catalyzed hydration of 2-pheny1propene is
Text Solution
|
- The best method to prepare cyclohexene form cyclohexanol is by using:
Text Solution
|
- Consider the following compounds: (I) 1, 2-dihydroxy benzene (II)...
Text Solution
|
- The reaction of with HBr gives :
Text Solution
|