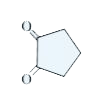
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - A|10 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - B|10 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE:3|8 Videos13-14 HYDROGEN & S-BLOCK ELEMENT
VMC MODULES ENGLISH|Exercise JEE Advanced Archive|48 VideosALCOHOLS, PHENOLS & ETHERS
VMC MODULES ENGLISH|Exercise Impeccable|100 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ALCOHOLS, PHENOLS & ETHERS -PRACTICE EXERCISE:4
- "Glycerol"overset(KHSO(4))toAoverset(LiAlH(4))toB. A and B are :
Text Solution
|
- Allyl alcohol is obtained when glycerol reacts with at 260^(@)C
Text Solution
|
- The oxidation product of 1,2-cyclopentane diol with HIO(4) or (CH(3)CO...
Text Solution
|
- When ethylene glycol is heated with a mixture of concentrated HNO(3) a...
Text Solution
|
- When glycerol is treated with excess of HI, it produces -
Text Solution
|
- When glycerol is heate with oxalic acid at 503 K, it produces :
Text Solution
|
- Glycerine is used in car radiators to :
Text Solution
|