A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-MOCK TEST 7-CHEMISTRY (SECTION 2)
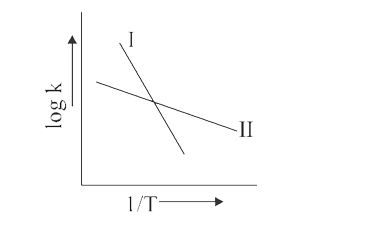
- The Arrhenius relationship of two different reaction is shown below: ...
Text Solution
|
- A solution containing H^(+) and D^(+) ions is in equilibrium with a mi...
Text Solution
|
- A gas (C(v,m)=(5)/(2)R) behaving ideally was allowed to expand reversi...
Text Solution
|
- 2.0g of a sample contains mixture of SiO2 and Fe2O3. On very strong he...
Text Solution
|
- Find the total number of monohalogenated products including stereoisom...
Text Solution
|
- Find the sum of X, Y and Z, here X is O – N – O bond angle in NO(3)^(-...
Text Solution
|