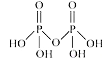
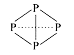A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-MOCK TEST 8-CHEMISTRY (SECTION 2)
- The number of sigma -bonds between P and O atom in H(4)P(2)O(7) and n...
Text Solution
|
- Consider the following cell reaction 2Fe((s))+O(2(g))+4H((aq.))^(o+)...
Text Solution
|
- What is the edge length of a cube whose volume is 4,096 cm^(3) ?
Text Solution
|
- When the following aldohexose exists in its D-configuration, the total...
Text Solution
|
- For an element, Cp = 23 + 0.01 T("JK"^(-1)"mol"^(-1)) . If temperatur...
Text Solution
|
- The spin only magnetic moment value (in Bohr magneton unit) of [Cr(CO)...
Text Solution
|