A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-MOCK TEST 11-CHEMISTRY (Section-2)
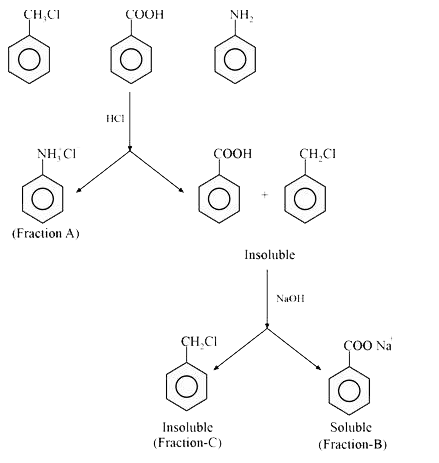
- A solution of Benzyl chloride, Benzoic acid and Aniline was extracted ...
Text Solution
|
- The number of chiral carbons in Ibuprofen "."
Text Solution
|
- If 200 ml of 0.031 M solution of H (2) SO(4) is added to 84 ml of a 0....
Text Solution
|
- Strontium 90 is one of the harmful nuclides resulting from nuclear fis...
Text Solution
|
- For the reaction, 2A (s) + 2B (g)to C (g) + D(s) at 298K Delta U ^(@...
Text Solution
|
- Chlorine reacts with hot and conc. Ba (OH)(2) and produces compound X,...
Text Solution
|