A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-MOCK TEST 13-CHEMISTRY ( SECTION-2)
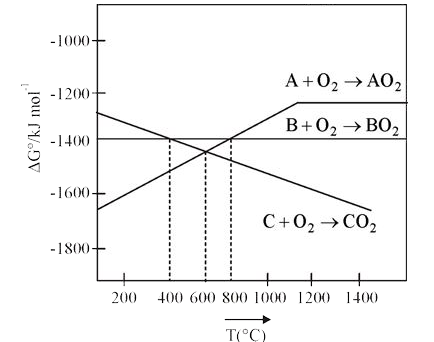
- According to the following diagram, which of the following option is c...
Text Solution
|
- The hardness of water sample ( in term of equivalengt of CaCO(3) ) co...
Text Solution
|
- The mass percentage of nitrogen in histidine is "". [Atomic mass H...
Text Solution
|
- The normality of H(2)SO(4) in a sample which has density 1.5 g//mL and...
Text Solution
|
- Electrolysis of dilute aqueous NaCl solution was carried out by passin...
Text Solution
|
- How much amount of NaCl in g should be added to900 g of water ( rho =1...
Text Solution
|