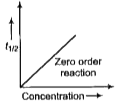
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- At 500K, the half-life period of a gaseous reaction at an initial pres...
Text Solution
|
- If a is the initial concentration of reaction, then the half life peri...
Text Solution
|
- At 500 K, the half-life period of a gaseous reaction at the initial pr...
Text Solution
|
- the decomposition of ammonia on tungsten surface at 500K follows zero ...
Text Solution
|
- At 500 K, the half-life period of a gaseous reaction at the initial pr...
Text Solution
|
- At 300 K, half life of a gaseous reactant initially at 58 KPa is 320 m...
Text Solution
|
- At 500K, the half period of a gaseous reaction at an initial pressure ...
Text Solution
|
- 500 K पर, एक गैसीय अभिक्रिया का अर्द्ध आयु काल 80 kPa के प्रारंभिक दाब...
Text Solution
|
- At 500K, the half-life period of a gaseous reaction at an initial pres...
Text Solution
|