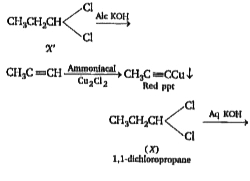
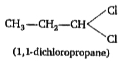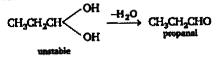A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A dihalogen derivative X of a hydrocarbon witn three carbon atom react...
Text Solution
|
- A dihalogen derivative (A) of a hydrocarbon having two carbon atoms re...
Text Solution
|
- A hydrocarbon reacts with HI to give X which on reaction with aqueous ...
Text Solution
|
- A dihalogen derivative 'X' of hydrocarbon with three carbon atom react...
Text Solution
|
- A dihalogen dervative 'X' of a hydrocarbon with three carbon atoms rea...
Text Solution
|
- A dihalogen derivative 'X' of a hydrocarbon with three carbon atoms re...
Text Solution
|
- A dihalogen derivative (A) with three C-atoms gives a hydrocarbon (B) ...
Text Solution
|
- The unsaturated hydrocarbon obtained by the reaction of a dihalogen de...
Text Solution
|
- The unsaturated hydrocarbon obtained by the reaction of a dihalogen de...
Text Solution
|