A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which set of terms correctly identifies the carbohydreate shown > ...
Text Solution
|
- Which set of terms correctly identifies the carbohydrate shown. 1. Pen...
Text Solution
|
- Which set of terms correctly identifies the carbohydrate shown ? ...
Text Solution
|
- Carbohydrates which differ in configurartion at the glycosidic carbon...
Text Solution
|
- In the given structure of the carbonhydrate, which of the following ar...
Text Solution
|
- পাইরানোজ ও ফিউরানোজ কাদের বলে ?
Text Solution
|
- How many aldose, ketose and furanose and pyranose units are present in...
Text Solution
|
- If number of aldose, ketose, furanose and pyranose units present in ma...
Text Solution
|
- Carbohydrates which differ in configuration at the glycosidic carbon (...
Text Solution
|

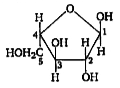 This compound contains five carbon atoms , so it is a pentose . Its first carbon contains -H and -OH group . This suggests that it is an aldoes (ie , contains aldehyde group). Since its structure is similar to furan (a heterocylic compound ) , so it has furanose structure.
This compound contains five carbon atoms , so it is a pentose . Its first carbon contains -H and -OH group . This suggests that it is an aldoes (ie , contains aldehyde group). Since its structure is similar to furan (a heterocylic compound ) , so it has furanose structure.